

The origin of quantum mechanics. Chronology of Quantum Mechanics, Molecular, Atomic, Nuclear, and Particle Physics. Timeline of atomic and subatomic physics. History of quantum mechanics. 10 influential figures in the history of quantum mechanics.
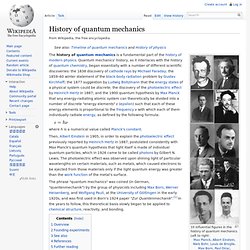
Left to right: Max Planck, Albert Einstein,Niels Bohr, Louis de Broglie,Max Born, Paul Dirac,Werner Heisenberg, Wolfgang Pauli,Erwin Schrödinger, Richard Feynman. The history of quantum mechanics is a fundamental part of the history of modern physics. Where h is a numerical value called Planck's constant. Then, Albert Einstein in 1905, in order to explain the photoelectric effect previously reported by Heinrich Hertz in 1887, postulated consistently with Max Planck's quantum hypothesis that light itself is made of individual quantum particles, which in 1926 came to be called photons by Gilbert N. Overview[edit] Ludwig Boltzmann's diagram of the I2 molecule proposed in 1898 showing the atomic "sensitive region" (α, β) of overlap. where: h is the Planck constant; Timeline of quantum mechanics. From Wikipedia, the free encyclopedia This timeline of quantum mechanics shows the key steps, precursors and contributors to the development of quantum mechanics, quantum field theories and quantum chemistry.[1][2] 19th century[edit] 1859 – Gustav Kirchhoff introduces the concept of a blackbody and proves that its emission spectrum depends only on its temperature.[1]1860–1900 – Ludwig Eduard Boltzmann, James Clerk Maxwell and others develop the theory of statistical mechanics. 20th century[edit] 1900–1909[edit] 1910–1919[edit] A schematic diagram of the apparatus for Millikan's refined oil drop experiment. 1920–1929[edit]
Overview. File:Bohr model 3.jpg. File:Boltzmanns-molecule.jpg. File:Feynmann Diagram Gluon Radiation.svg. Cancel Edit Delete Preview revert Text of the note (may include Wiki markup) Could not save your note (edit conflict or other problem).
Please copy the text in the edit box below and insert it manually by editing this page. Upon submitting the note will be published multi-licensed under the terms of the CC-BY-SA-3.0 license and of the GFDL, versions 1.2, 1.3, or any later version. See our terms of use for more details. Add a note Draw a rectangle onto the image above (press the left mouse button, then drag and release). Save To modify annotations, your browser needs to have the XMLHttpRequest object. [[MediaWiki talk:Gadget-ImageAnnotator.js|Adding image note]]$1 [[MediaWiki talk:Gadget-ImageAnnotator.js|Changing image note]]$1 [[MediaWiki talk:Gadget-ImageAnnotator.js|Removing image note]]$1.