

Dollars for Docs - ProPublica. University of Cambridge launches Open Innovation drug discovery initiative with GSK. The University of Cambridge is embarking on a programme of scientific open collaboration with GlaxoSmithKline (GSK) that will involve working alongside GSK scientists and other partner organisations, to advance drug discovery and the development of new medicines.
Researchers will be based at Stevenage Bioscience Catalyst, the UK’s first open innovation bioscience campus, co-located with GSK’s Research and Development centre. This bioscience park provides an independent scientific community allowing scientists to foster relationships across organisations... This page requires a Science|Business Network Membership This page is available to Science|Business Network Members only. If you are already a member then please log in (above). Note if you are only registered to receive our Bulletin, or have previously purchased an item from our store, this does not give you access to the full benefits of the site. Be where innovation begins – become a member. GlaxoSmithKline pay $3b fine after pleading guilty to healthcare fraud.
By Daily Mail Reporter Published: 16:14 GMT, 2 July 2012 | Updated: 00:35 GMT, 3 July 2012 A UK drugs firm has been hit with a $3billion penalty after admitting to the ‘biggest healthcare fraud in history’.

GlaxoSmithKline to Pay $3 Billion for Diabetes Drug Scam. GlaxoSmithKline Found Guilty of Drug Fraud Settlement. DUB: ‘Innovatie van geneesmiddelen heeft commerciële drive nodig’ Webarchive: DUB ‘Innovatie van geneesmiddelen heeft commerciële drive nodig’ Catalogus Professorum - Prof Detail. Catalogus Prowebarchive: fessorum - Prof Detail. Research Desk - dhr. prof. dr. J.A.M. Raaijmakers. Research Desk - dhr. prof. dr. J.A.M. Raaijmakers.
Pharmaceutical Sciences - Pharmacoepidemiology and Pharmacotherapy. Utrecht Institute for Pharmaceutical Sciences Phone : +31 (0)30 253 7324 E-mail : science.officeff@uu.nl About the division The division of Pharmacoepidemiology & Clinical pharmacology consists of a multidisciplinary team of young and internationally oriented researchers.
The research program is directed at several epidemiological, therapeutic, and policy aspects of chronic drug use, especially focusing on anti-asthmatics, cardiovascular drugs, and psychotropics. The internationally acclaimed Pharmo record linkage system was initially developed at the division, in close collaboration with the Erasmus University of Rotterdam. Population-based studies of drug treatment: from molecule to patient outcomes With the prospect that innovative drug therapies will be introduced in the coming years, society demands new approaches and concepts for comparative risk/benefit evaluation. UCLA tells professors not to apply for major new pharmaceutical grant. These days many research universities are constantly looking for new grant competitions and encouraging their faculty members to apply.
On Friday, the University of California at Los Angeles took the unusual step of telling professors not to apply to a major new grant competition from a pharmaceutical company, saying that the program violated university rules. An e-mail marked "urgent" was sent Friday to all faculty members and deans about the Discovery Fast Track Competition, which was just announced this month and for which the sponsor -- GlaxoSmithKline -- is approaching faculty members directly, bypassing technology transfer offices at universities. The company announced that the program was an attempt to reward academic researchers by offering a "fast track" to financing their most creative ideas. Faculty members are invited to submit short proposals and promised a quick decision later this year, leading to funding.
GlaxoSmithKline 'the big boss' in £300m bribery scandal, China says. GlaxoSmithKline research centre in Shanghai.
The company is under investigation over alleged bribery. Photograph: Yang Shichao/ Yang Shichao/Xinhua Press/Corbis China has accused the British pharmaceutical company GlaxoSmithKline (GSK) of behaving like a criminal godfather, bribing doctors with cash and sexual favours in return for prescribing its drugs. Chinese police have detained four senior Chinese executives as part of an investigation that stretches back to 2007 and involves deals worth 3bn yuan (£320m). The Chinese investigator leading the inquiry said the head of GSK's Chinese operations, Mark Reilly, a British national, had left the country on 27 June. Banker bonuses for Glaxo boffins. Sir Andrew Witty is confident the approach will work (Tom Stockill) SCIENTISTS at Britain’s biggest drug company are in line for banker-style bonuses if the medicines they discover make it to market.
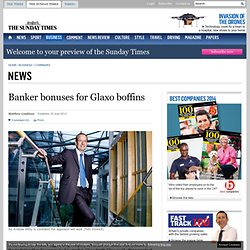
Glaxo Smith Kline could offer payouts of between £5m and £10m a drug. The company will determine who is entitled to share in the payout by judging which staff were key to its discovery and development. The awards are part of efforts by Glaxo to shake up its research and development (R&D) effort. In the past, scientists were incentivised according to the various stages of development, such as the discovery of a new molecule or the first time it was tested on humans. This year, Glaxo has had three new treatments approved by health watchdogs in America, the biggest drug market, including two cancer medicines and. Muscular dystrophy drug from GlaxoSmithKline, Prosensa fails PhIII. GlaxoSmithKline and Prosensa today conceded defeat in the Phase III study for drisapersen, one of two closely-watched therapies for Duchenne muscular dystrophy which had been vying for the lead in the field.
Investigators reported that the therapy failed to significantly improve walking distance in patients. Rudolf van Olden (rwvanolden) Medical director GSK... Gemeente de ronde venen. VVD De Ronde Venen > Gemeenteraadsfractie. Image g1FS. Image 9I5G. Rudolf van Olden (1746-1813). Raad- en rekenmeester van prins Willem V, Leonardus Temminck, 1780 - 1800. Ger Van Olden (gervanolden) sur Twitter. Huib van olden (huibvanolden) sur Twitter. Announces changes to its global sales and marketing practices to further ensure patient interests come first. Company to roll out new sales force compensation programme, removing individual sales targetsBegins process to end direct payments to healthcare professionals for speaking engagements or attendance at medical conferences by start of 2016 Issued: Tuesday 17 December 2013, London UK GSK today set out plans to evolve the way it sells and markets its products to healthcare professionals to further align the company’s activities with the interests of patients.
During 2014, the company will implement a new compensation system which will apply to all GSK sales employees who work directly with prescribing healthcare professionals. The company also intends to begin a consultative process towards stopping direct payments to healthcare professionals for speaking engagements and for attendance at medical conferences. Global sales force compensation changes The new compensation programme will have no individual sales targets. Payments to healthcare professionals. Glaxo: the new modern face of big pharma? The rules of retraction. Years after the antidepressant Seroxat was banned for under-18s, Melanie Newman reports on calls for withdrawal of a paper supporting its use in adolescents Years after regulators banned GlaxoSmithKline’s antidepressant paroxetine (Seroxat) for under-18s, two academics are fighting for a paper claiming the drug is safe and effective for adolescents to be withdrawn.
The 2001 paper in the Journal of the American Academy of Child and Adolescent Psychiatry (JAACAP)1 concludes that paroxetine is “generally well tolerated and effective” for treatment of major depression in adolescents. The paper gives a misleading impression of the trial’s results and the journal should retract it, say Jon Jureidini, associate professor of psychiatry at the University of Adelaide, and Leemon McHenry, lecturer in philosophy at California State University.
The efficacy claim was based on just 15% of the trial’s outcomes, they argue. A recent Thomson Reuters analysis … (pdf) The rules of retraction The BMJ. Study 329. Study 329 was a clinical trial conducted in North America from 1994 to 1998 to study the efficacy of paroxetine, an SSRI anti-depressant marketed as Paxil and Seroxat, in treating depressed teenagers.[1] Paroxetine was released in 1991 by the British pharmaceutical company SmithKline Beecham, known since 2000 as GlaxoSmithKline (GSK).
The drug made $11.6 billion between 1997 and 2006.[2] Study 329, which compared paroxetine with imipramine, a tricyclic antidepressant marketed as Tofranil, failed to show efficacy for paroxetine in adolescent depression, something SmithKline Beecham acknowledged internally in 1998. [n 1] In addition there were more examples of suicidal thinking and behaviour in the group taking paroxetine. [n 2] Although the article included these negative results, it did not account for them in its conclusion.
On the contrary, it concluded that paroxetine is "generally well tolerated and effective for major depression in adolescents Clinical trial[edit] JAACAP article[edit] Producent antidepressivum verzweeg schadelijke gevolgen voor jongeren.