

ATSDR Home. Lead_poisoning.pdf (application/pdf Object) GLYPHOSATE:Deadly to Human Cells Deadlier then DDT at Candida Support Forum (MessageID: 1907004) Please try to buy organic or pesticide free or raise your own veg.s....
Monsanto Land was Poisoned and the Barren Women Cried Human Fertility Today Scores Less than 50% of Levels 20 years ago So what has changed in our environment to affect mankind's fertility in such a drastic manner, and please, be assured that this is a presently occurring catastrophe. I follow a guy called Dr.Mercola,(USA) who has one of the world's leading natural health websites.He recently had an interview with DR HUBER,who is an expert in an area of Science that relates to the toxicity of genetically engineered (GE) foods.His specific areas of training include soil bourne diseases, microbial ecology, and host-parasite relationships.Dr.
Huber also taught plant pathology, soil microbiology, and micro-ecological interactions, as a staff Professor at Purdue University for 35 years, eminently expert and qualified. Dr.Huber has observed the effects of GM at work. This is a global problem. What must we do? Trichloroethylene. The chemical compound trichloroethylene (C 2HCl 3) is a chlorinated hydrocarbon commonly used as an industrial solvent.
It is a clear non-flammable liquid with a sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene. The IUPAC name is trichloroethene. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. It has been sold under a variety of trade names. History[edit] Pioneered by Imperial Chemical Industries in Britain, its development was hailed as an anesthetic revolution. The introduction of halothane in 1956 greatly diminished the use of TCE as a general anesthetic.
Due to concerns about its toxicity, the use of trichloroethylene in the food and pharmaceutical industries has been banned in much of the world since the 1970s. Groundwater contamination by TCE has become an important environmental concern for human exposure. Production[edit] HC≡CH + 2 Cl2 → Cl2CHCHCl2 Cl2CHCHCl2 → ClCH=CCl2 + HCl. 42_3_LAS%20VEGAS_09-97_0807. Bis(2-ethylhexyl) phthalate. Bis(2-ethylhexyl) phthalate (di-2-ethylhexyl phthalate, diethylhexyl phthalate, DEHP; dioctyl phthalate, DOP), is an organic compound with the formula C6H4(C8H17COO)2.
DEHP is the most common of the class of phthalates which are used as plasticizers, accounting for an almost 54% market share in 2010.[1] It is the diester of phthalic acid and the branched-chain 2-ethylhexanol. This colourless viscous liquid is soluble in oil, but not in water. DEHP is a High Production Volume Chemical causing widespread, global human exposure. Phthalates are chemicals of concern, because they are endocrine disruptors. The 3 stereoisomers of DEHP Due to its suitable properties and the low cost, DEHP is widely used as a plasticizer in manufacturing of articles made of PVC.[2] Plastics may contain 1% to 40% of DEHP. Industrial production entails the reaction of phthalic anhydride with 2-ethylhexanol: Bisphenol A.
Bisphenol A (BPA) is a carbon-based synthetic compound with the chemical formula (CH3)2C(C6H4OH)2 belonging to the group of diphenylmethane derivatives and bisphenols.
It has been in commercial use since 1957. BPA is employed to make certain plastics and epoxy resins. BPA-based plastic is clear and tough, and is made into a variety of common consumer goods, such as baby and water bottles, sports equipment, CDs, and DVDs. Epoxy resins containing BPA are used to line water pipes, as coatings on the inside of many food and beverage cans and in making thermal paper such as that used in sales receipts. BPA is part of the bisphenols group of chemical compounds with two hydroxyphenyl groups. BPA exhibits hormone-like properties at high dosage levels that raise concern about its suitability in consumer products and food containers where exposure is orders of magnitude lower. Production[edit] A large number of ketones undergo analogous condensation reactions. Use[edit] History[edit] Roundup (herbicide) Glyphosate (N-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide used to kill weeds, especially annual broadleaf weeds and grasses known to compete with commercial crops grown around the globe.
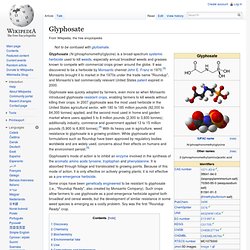
It was discovered to be a herbicide by Monsanto chemist John E. Franz in 1970.[3] Monsanto brought it to market in the 1970s under the trade name "Roundup", and Monsanto's last commercially relevant United States patent expired in 2000. Glyphosate was quickly adopted by farmers, even more so when Monsanto introduced glyphosate-resistant crops, enabling farmers to kill weeds without killing their crops. Glyphosate's mode of action is to inhibit an enzyme involved in the synthesis of the aromatic amino acids tyrosine, tryptophan and phenylalanine. It is absorbed through foliage and translocated to growing points.
Some crops have been genetically engineered to be resistant to glyphosate (i.e., "Roundup Ready", also created by Monsanto Company). Discovery[edit]