

Learn about Periodic Table of Elements. Periodic Table Video. The Periodic Table. The periodic table is a way to organize the elements that create the chemicals that form every physical thing in the world.
Understand how the periodic table is organized with important facts from a science teacher in this free video on fundamental physics. Now let's get into the organization of matter. To really understand how matter and what we as human beings know thus far what exists in matter we have to talk about the periodic table of elements. One has to realize that every substance in the universe is made up of chemicals such as hydrogen, helium, and oxygen. Thus all the chemicals are made up of elements. Don’t miss: Slideshows. Visual Elements: Patterns in the Periodic Table. Patterns in the Periodic Table The periodic table is an arrangement of all the known elements in order of increasing atomic number.
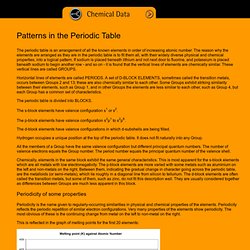
The reason why the elements are arranged as they are in the periodic table is to fit them all, with their widely diverse physical and chemical properties, into a logical pattern. If sodium is placed beneath lithium and not next door to fluorine, and potassium is placed beneath sodium to begin another row - and so on - it is found that the vertical lines of elements are chemically similar. These vertical lines are called GROUPS. Horizontal lines of elements are called PERIODS. The periodic table is divided into BLOCKS. The s-block elements have valence configuration s1 or s2. The p-block elements have valence configuration s2p1 to s2p6.
The d-block elements have valence configurations in which d-subshells are being filled. Hydrogen occupies a unique position at the top of the periodic table. Periodicity of some properties. Dynamic Periodic Table. Bill Nye - Atoms 1. All About Atoms - What are atoms? How Atoms Work" It has been said that during the 20th century, man harnessed the power of the atom.
We made atomic bombs and generated electricity by nuclear power. We even split the atom into smaller pieces called subatomic particles. But what exactly is an atom? What is it made of? What does it look like? All matter is made up of tiny particles called atoms. All matter is composed of extremely small particles called atoms.

All atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements can combine in simple, whole-number ratios to form compounds. In chemical reactions, atoms are combined, separated, or rearranged. Adjustments to Dalton's Atomic Theory We now know that atoms of the same element can have different masses and are called isotopes.
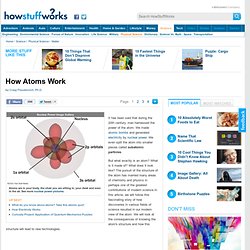
We have also discovered the possibility of atomic fission; splitting the atom (Hiroshima & Nagasaki). Elements are composed of atoms, which are extremely small. Def: An atom is the smallest unit of a substance that retains the properties of that substance. Atoms are composed of three types of particles: Rutherford's view of the atom (1914) Bohr models of various atoms. (1920) Quantum model of a sodium atom. Pictures of Atomic Orbitals. Energy Levels.