Primary Metallic Crystalline Structures. Primary Metallic Crystalline Structures (BCC, FCC, HCP) As pointed out on the previous page, there are 14 different types of crystal unit cell structures or lattices are found in nature.
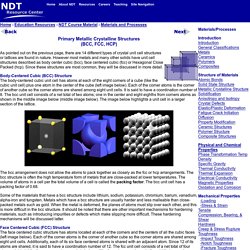
However most metals and many other solids have unit cell structures described as body center cubic (bcc), face centered cubic (fcc) or Hexagonal Close Packed (hcp). Since these structures are most common, they will be discussed in more detail. Body-Centered Cubic (BCC) Structure The body-centered cubic unit cell has atoms at each of the eight corners of a cube (like the cubic unit cell) plus one atom in the center of the cube (left image below). Fiberglass Manufacturing How Fiberglass Is Made. The Basics of Fiberglass Fabric. P1. Material Structures. Atomic Structure of Materials. Structure of Materials.
Structure of Materials It should be clear that all matter is made of atoms.
From the periodic table, it can be seen that there are only about 100 different kinds of atoms in the entire Universe. These same 100 atoms form thousands of different substances ranging from the air we breathe to the metal used to support tall buildings. Body Centered Cubic Crystal Lattice. BODY CENTERED CUBIC (bcc) Click on the unit cell to view it rotating.

We can think of this unit cell as made by stuffing another atom into the center of the simple cubic lattice, slightly spreading the corners. Thus, the corner spheres no longer quite touch one another, but do touch the center. The diagonal through the body of the cube is 4x (sphere radius). The packing efficiency of a bcc lattice is considerably higher than that of a simple cubic: 69.02 % The higher coordination number and packing efficency mean that this lattice uses space more efficiently than simple cubic. The unit cells stack together like this: Learn About Ceramics: Structure and Properties of Ceramics. May 21st, 2014 Published on May 21st, 2014 | By: pwray@ceramics.org The properties of ceramic materials, like all materials, are dictated by the types of atoms present, the types of bonding between the atoms, and the way the atoms are packed together.

The type of bonding and structure helps determine what type of properties a material will have. Ceramics usually have a combination of stronger bonds called ionic (occurs between a metal and nonmetal and involves the attraction of opposite charges when electrons are transferred from the metal to the nonmetal); and covalent (occurs between two nonmetals and involves sharing of atoms). Ceramics - Chemistry Encyclopedia - structure, water, uses, elements, examples, metal, number, salt. Photo by: Acik Ceramics can be defined as heat-resistant, nonmetallic, inorganic solids that are (generally) made up of compounds formed from metallic and nonmetallic elements.
Although different types of ceramics can have very different properties, in general ceramics are corrosion-resistant and hard, but brittle. Most ceramics are also good insulators and can withstand high temperatures. These properties have led to their use in virtually every aspect of modern life. The two main categories of ceramics are traditional and advanced. Glass is sometimes considered a type of ceramic. Composition Some ceramics are composed of only two elements. Ceramics are good insulators and can withstand high temperatures. Silicon dioxide, SiO 2 .
Polymer Structure. Polymer Structure Engineering polymers include natural materials such as rubber and synthetic materials such as plastics and elastomers.

Polymers are very useful materials because their structures can be altered and tailored to produce materials 1) with a range of mechanical properties 2) in a wide spectrum of colors and 3) with different transparent properties. Mers A polymer is composed of many simple molecules that are repeating structural units called monomers. A single polymer molecule may consist of hundreds to a million monomers and may have a linear, branched, or network structure. Covalent bonds hold the atoms in the polymer molecules together and secondary bonds then hold groups of polymer chains together to form the polymeric material. Polymer Chains (Thermoplastics and Thermosets) A polymer is an organic material and the backbone of every organic material is a chain of carbon atoms. The ability for molecules to form long chains is a vital to producing polymers.
Chapter 15. Polymer Structures. 15.1 Introduction Polymers are common in nature, in the form of wood, rubber, cotton, leather, wood, silk, proteins, enzymes, starches, cellulose.

Artificial polymers are made mostly from oil. Their use has grown exponentially, especially after WW2. The key factor is the very low production cost and useful properties (e.g., combination of transparency and flexibility, long elongation). 15.2 Hydrocarbon Molecules Most polymers are organic, and formed from hydrocarbon molecules. Isomers are molecules that contain the same molecules but in a different arrangement. 15.3 Polymer Molecules Polymer molecules are huge, macromolecules that have internal covalent bonds. Tecnoelpalo - information about plastics. Skip to main content Get your brand new Wikispaces Classroom now and do "back to school" in style. guest Join | Help | Sign In tecnoelpalo Home guest| Join | Help | Sign In Turn off "Getting Started" Loading...