

No more tea stains and chalky deposits. Dishwashing hands were something Josephine Cochrane had only heard about,being a well-to-do American lady with servants to perform such chores.

Unfortunately, pieces of her beloved porcelain repeatedly got broken. So Cochrane took the initiative and invented a dishwashing machine in which her valuable porcelain service was secured in wire compartments and rinsed with hot, soapy water. Her device was patented in 1886 and is regarded as the world’s first practical dishwasher. These machines were still cumbersome, however, and did not become widely adopted for home use until the 1960s when the first fully automated versions with a rinse cycle were developed. Teknokemian Yhdistys. Norrex oy - teknokemia - raisio (turku) Micoltech - teknokemian tutkimus - turku.
JL-Tuotteet Oy - pesuaineet - tampere. Berner - teknokemia - heinävesi, rajamäki, helsinki. Noso-Tuote Oy - puhdistus ain. - tuusula. Farmos + kiilto. Suomalaisten korkeatasoisten elintarvikkeiden lähtökohtia ovat turvallisuus, puhtaus ja maukkaus sekä pellolta pöytään kulkeva laatuketju.

Alkutuotannon hygienia takaa laadukkaan lopputuloksen, sillä raaka-aineiden puhtautta ei voida korvata missään muussa elintarvikeketjun vaiheessa. Farmos - turku. Kemvit Oy - pesunest. ja -jauh. - alaveteli (kokkola) Kemvit Oy on 100 %:sti suomalaisessa omistuksessa oleva yritys, joka valmistaa pesujauheita teollisuuteen ja kuluttajille sekä maatalouteen.
Yrityksellä on valmius kehittää ja valmistaa puhdistusaineita kaikkiin tarkoituksiin, sekä nestemäisinä että jauheina, asiakkaan toivomusten ja tarpeiden mukaan. Alfa kem Oy Ab - puhtaanapitoaineet - lahti? Oy Alfa-Kem Ab on perustettu vuonna 1982 palvelemaan teknokemian tuotteiden ammattikäyttäjiä teollisuudessa, elintarviketiloissa ja siivouspalveluissa.
Refinery Products. Arizona Chemical provides tall oil fatty acids, distilled tall oils, tall oil rosins and crude sulphate turpentine derived products to the global coatings, inks, fuel additives and lubricants industries.

Biosurfactant. Schematic diagram of a micelle of oil in aqueous suspension, such as might occur in an emulsion of oil in water.
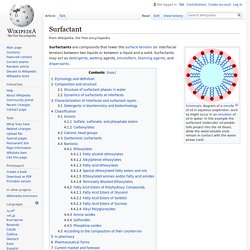
In this example the surfactant molecules' oil-soluble tails project into the oil (blue), while the water-soluble ends remain in contact with the water phase (red). Surfactants are compounds that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants. Etymology and definition[edit] The term surfactant is a blend of surface active agent.[1] In the United States National Library of Medicine's Medical Subject Headings (MeSH) vocabulary, surfactant is reserved for the meaning pulmonary surfactant.
Schematic diagram of a micelle – the lipophilic tails of the surfactant ions remain inside the oil because they interact more strongly with oil than with water. Composition and structure[edit] Textiles - Genencor a Danisco division - hanko. AB Enzymes - Technical Enzymes - rajamäki (hyvinkää) Tiede kosmetiikan takana.
Pesuaktiiviset aineet. CLEANRIGHT. Welcome to our ingredients database, in which you will find information on ingredients commonly found in soaps, detergents and maintenance products.
To find an ingredient, click on the first letter of its name in the A-Z selection range above. A list will appear below in alphabetical order. Simply scroll down to the desired ingredient to obtain information on the type(s) of product in which the ingredient is used, the family group, CAS number*, safety information, as well as links to further information.
Please note that this list is not exhaustive and will be completed overtime. It provides links to safety information available in the public domain which is also coupled by other data and risk assessments conducted by individual companies.Please be aware that the links below are only available in English. * CAS Number :A CAS (Chemical Abstract Service index number)registry number is a unique numerical identifier. For information on fragrances/perfume ingredients, please click here. CLEANRIGHT - compounds. BioUltra Reagents Detergents & Surfactants. Surfactant. How Detergents Work - Chemistry of Surfactants. Question: How Do Detergents Clean?
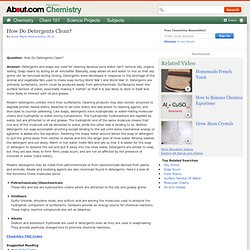
Answer: Detergents and soaps are used for cleaning because pure water can't remove oily, organic soiling. Soap cleans by acting as an emulsifier. Basically, soap allows oil and water to mix so that oily grime can be removed during rinsing. Detergents were developed in response to the shortage of the animal and vegetable fats used to make soap during World War I and World War II. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Modern detergents contain more than surfactants. Modern detergents may be made from petrochemicals or from oleochemicals derived from plants and animals. Petrochemicals/OleochemicalsThese fats and oils are hydrocarbon chains which are attracted to the oily and greasy grime.OxidizersSulfur trioxide, ethylene oxide, and sulfuric acid are among the molecules used to produce the hydrophilic component of surfactants.
Chemistry FAQ Index. Detergent. Bile Salts - Intestinal Natural Detergents: Bile acids are produced in the liver and secreted in the intestine via the gall bladder.
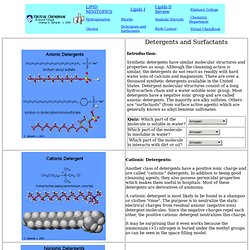
Bile acids are oxidation products of cholesterol. First the cholesterol is converted to the trihydroxy derivative containing three alcohol groups. The end of the alkane chain at C # 17 is converted into an acid, and finally the amino acid, glycine is bonded through an amide bond. The acid group on the glycine is converted to a salt. The main function of bile salts is to act as a soap or detergent in the digestive processes. Soap. A collection of decorative soaps, often found in hotels Two equivalent images of the chemical structure of sodium stearate, a typical soap.
Mechanism of cleansing soaps[edit] Structure of a micelle, a cell-like structure formed by the aggregation of soap subunits (such as sodium stearate): The exterior of the micelle is hydrophilic (attracted to water) and the interior is lipophilic (attracted to oils). Action of soap[edit] When used for cleaning, soap allows otherwise insoluble particles to become soluble in water and then be rinsed away.
Effect of the alkali[edit] Chemistry in your cupboard. Linkit. Erisat. Sertot. Pakkausmerkinnät. A.I.S.E. - coming labeling (fin?)