

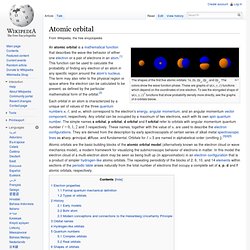
The Art of Electronics. Atomic orbital. The shapes of the first five atomic orbitals: 1s, 2s, 2px, 2py, and 2pz.
Molecular orbital. Complete acetylene (H–C≡C–H) molecular orbital set.
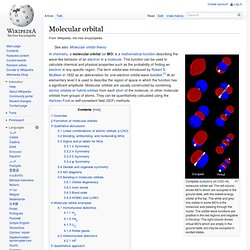
The left column shows MO's which are occupied in the ground state, with the lowest-energy orbital at the top. The white and grey line visible in some MO's is the molecular axis passing through the nuclei. Electron configuration. Electron atomic and molecular orbitals In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.[1] For example, the electron configuration of the neon atom is 1s2 2s2 2p6.
Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions. Valence. Valence or valency may refer to: Science[edit] In several fields the valence of an element refers to the number of elements to which it can connect: Places[edit] France[edit] Valence, Charente, a commune in the Charente departmentValence, Drôme, a commune and prefecture of the Drôme department University of Valence, a medieval universityValence, Tarn-et-Garonne, a commune in the Tarn-et-Garonne departmentCanton of Valence, Tarn-et-Garonne departmentArrondissement of Valence, Drôme departmentRoman Catholic Diocese of ValenceValence-d'Albigeois, in the Tarn departmentValence-en-Brie, in the Seine-et-Marne departmentValence-sur-Baïse, in the Gers department England[edit] River Valency, Cornwall.
Covalent bond. A covalent bond forming H2 (right) where two hydrogen atoms share the two electrons A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.
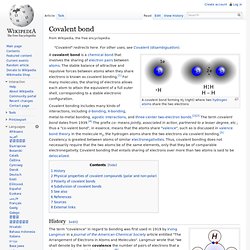
The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.[1] For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration. Valence (chemistry) In chemistry, valence, also known as valency or valence number, is defined by the IUPAC as:- The maximum number of univalent atoms (originally hydrogen or chlorine atoms) that may combine with an atom of the element under consideration, or with a fragment, or for which an atom of this element can be substituted. .[1] An alternative modern description is:-[2] The number of hydrogen atoms that can combine with an element in a binary hydride or twice the number of oxygen atoms combining with an element in its oxide or oxides.
This definition differs from the IUPAC definition as an element can be said to have more than one valence. Valence bond theory. In chemistry, valence bond (VB) theory is one of two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding.
It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule.[1] History[edit] Theory[edit] The overlapping atomic orbitals can differ. VB theory today[edit] Valence bond theory now complements molecular orbital (MO) theory, which does not adhere to the VB idea that electron pairs are localized between two specific atoms in a molecule but that they are distributed in sets of molecular orbitals which can extend over the entire molecule. Valence band. Valence (psychology) The term entered English usage in psychology with the translation from German in 1935 of works of Kurt Lewin.
Ambivalence has a longer history. [citation needed] Valence could be assigned a number and treated as if it were measured, but the validity of a measurement based on a subjective report is questionable. Measurement based on observations of facial expressions, using the Facial Action Coding System and microexpressions (see Paul Ekman), or on modern functional brain imaging may overcome this objection. Jump up ^ Nico H. Valency (linguistics) There are several types of valency: impersonal (=avalent), intransitive (=monovalent), transitive (=divalent) and ditransitive (=trivalent): an impersonal verb takes no arguments, e.g.
It rains. (Though it is technically the subject of the verb in English, it is only a dummy subject, that is a syntactic placeholder - it has no concrete referent. No other subject can replace it. In many other languages, there would be no subject at all. The term valence also refers to the syntactic category of these elements. Winning the prize made our training worthwhile. - Subject is a non-finite verb phrase That he came late did not surprise us. - Subject is a clause Sam persuaded us to contribute to the cause. - Object is a non-finite verb phrase. Why Nikola Tesla was the greatest geek who ever lived. Additional notes from the author: If you want to learn more about Tesla, I highly recommend reading Tesla: Man Out of Time Also, this Badass of the week by Ben Thompson is what originally inspired me to write a comic about Tesla.

Ben's also got a book out which is packed full of awesome. There's an old movie from the 80s on Netflix Instant Queue right now about Tesla: The Secret of Nikola Tesla. It's corny and full of bad acting, but it paints a fairly accurate depiction of his life. The drunk history of Tesla is quite awesome, too. History.com has a great article about Edison and how his douchebaggery had a chokehold on American cinema. Getting_Started_in_Electronics_(Radio_Shack)_-_F._Mims_(1994)_WW.pdf.