

Welsh scientists make major Alzheimer’s breakthrough. Researchers from Wales have helped uncover 11 new genes linked with Alzheimer’s in a major breakthrough into the causes of the disease.
Scientists from Cardiff University jointly led an international collaboration gathering data from more than 74,000 people – the largest ever study of its kind – as part of a two-year project, the findings of which are published today. The academic group, headed by Merthyr Tydfil-born professor Julie Williams, has helped discover a record 11 new susceptibility genes linked with Alzheimer’s disease. It is said the breakthrough will significantly increase knowledge of the disease and lead to a better understanding of its disordered functional processes while throwing open new research avenues. Prof Williams said: “I would describe it as a significant step forward, really helping us understand what is causing the common form of Alzheimer’s disease. “This is why it has proved somewhat successful.” “They have been fantastic in their support.
Prion protein-mediated toxicity of amyloid-β oligomers requires lipid rafts and the transmembrane LRP1. - AlzForum Alzheimer Research Paper of the Week. We have hypothesized that Aβ oligomer binding to cellular prion protein (PrPC) plays a central role in the pathogenesis of Alzheimer’s disease (AD) (Gimbel et al., 2010; Lauren et al., 2009; Um et al., 2012).
Two recent papers, this one together with Fluharty et al., 2013, provide further evidence for a key role of PrPC in Aβ-mediated toxicity. Here, Hooper and colleagues show that specific assemblies of Aβ preferentially bind to PrPC, with downstream activation of the Src family kinase Fyn. We recently defined a signaling cascade by which Aβ activates Fyn and the NR2B subunit of the NMDA receptor in a PrPC-dependent fashion (Um et al., 2012). In addition, similar findings were recently reported by Larson et al. (Larson et al., 2012), providing strong converging evidence for a pathologic Aβ-PrPC-Fyn signaling cascade in AD models.
Here, Hooper and colleagues show that specific assemblies of Aβ preferentially bind to PrPC, with downstream activation of the Src family kinase Fyn. Membrane associated misfolded pro... [Neuropathol Appl Neurobiol. Like a prion, Alzheimer's protein seeds itself in the brain. The Alzheimer’s-related protein amyloid-beta is an infectious instigator in the brain, gradually contorting its harmless brethren into dangerous versions, new evidence suggests.
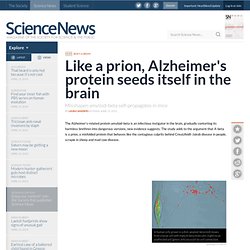
The study adds to the argument that A-beta is a prion, a misfolded protein that behaves like the contagious culprits behind Creutzfeldt-Jakob disease in people, scrapie in sheep and mad cow disease. In human cells grown in a dish, amyloid-beta (red) moves from a nerve cell with many A-beta molecules (right) to an unaffected cell (green, left) via a cell-to-cell connection.
Sangeeta Nath Amyloid-beta taken from the brain of one mouse was injected into a second mouse brain (shown); 300 days later, the protein had spread throughout and formed deposits (dark spots). J. Prion. Alzheimer’s disease: the silent prion (TSEs) - Dayton Biology. The protein, known as amyloid-beta, codes for the gene able to activate Alzheimer’s disease; this dangerous culprit (amyloid-beta) is a prion, which is a misfolded protein that is able to convert normal proteins into more prions.
When altering the shape of a protein, which creates a prion, the function of the protein is altered as well, creating an entirely different protein; changing the shape of a protein converts the protein into a completely new protein. In addition to Alzheimer’s disease, many scientists have been led to believe that A-beta also accounts for diseases such as Creutzfeldt-Jakob, scrapie, and mad cow. Although Alzheimer’s disease is incapable of spreading from person to person, being a prion disease, Alzheimer’s disease has the ability to mutate or “spread” internally. Currently, scientists are baffled as to what specific form of the A-beta protein is responsible for causing prion activity inside the brain.