

DISOLUCIONES. Se mezclan 5,00 g de cloruro de hidrógeno (HCI) con 35,00 g de agua, formándose una disolución cuya densidad a 20 ºC es de 1,060 g/cm3.

Calcúlese: a) El tanto por ciento en peso. b) La concentración en gramos por litro. c) La molaridad y d) La molalidad. a) Tanto por ciento. DISEÑO MECANICO DE RECIPIENTES A PRESION. El coste de un PCP. Muchos en su día cuando descubrimos eso que se denomina PCP quedamos maravillados por esa tecnología y se nos hacia la boca agua ante un mundo nuevo que nos brindaba nuevas posibilidades para el aire comprimido.

Conductividad del NaCl. Electrical resistivity and conductivity. Definition[edit] Resistors or conductors with uniform cross-section[edit] A piece of resistive material with electrical contacts on both ends. where R is the electrical resistance of a uniform specimen of the material (measured in ohms, Ω) is the length of the piece of material (measured in metres, m) A is the cross-sectional area of the specimen (measured in square metres, m2).
The reason resistivity is defined this way is that it makes resistivity an intrinsic property, unlike resistance. In a hydraulic analogy, passing current through a high-resistivity material is like pushing water through a pipe full of sand, while passing current through a low-resistivity material is like pushing water through an empty pipe. The above equation can be transposed to get Pouillet's law (named after Claude Pouillet): The resistance of a given material will increase with the length, but decrease with increasing cross-sectional area. The formula and General definition[edit] Conductivity is the inverse: [edit] Cálculo de caudal de agua en tubería. El cálculo del caudal de agua que recorre un conjunto de tuberías, que forman una red o un circuito, es importante para determinar las necesidades de energía que harán que el agua circule por ellas en las condiciones determinadas por el proyecto que se trate.
El conjunto de tuberías puede pretenecer a redes tanto en los edificios, como la de calefacción o la de agua corriente, como en la industria. Agua desionizada/desmineralizada. Search through over 10 million science, health, medical journal full text articles and books. Van der Waals and Nitrox. Revised VersionFull screen JOHANNES DIDERIK VAN DER WAALS 1837-1923 Amsterdam University 1910 Nobel Prize for Physics for his work on the equation of state for gases and liquids.

This tends to indicate that he knew his stuff. OK. The simple rules for gases about pressure, volume and temperature that you learnt in Scuba for beginners are only an approximation. Good enough for some poor lad or lass who has to worry about doing a mask clear without a total sinus washout but now you're grown up and want to breath fancy stuff it just won't do anymore. You haven't forgotten but we'll recap anyway. Mols? Great. Then along comes Van der Waals in spoil sport mode and says "well not really". The trouble is that this thing is the Ideal gas law. What he came up with was not the exact answer but a much better gas equation. Back to the example for our tank of O2 and use the Oxygen a value of 1.382 and the b value of 0.03186 and we get a different situation. It gets worse. What on Earth? Bit esoteric eh? Forward osmosis. Osmotic Membrane Processes Forward osmosis is an osmotic process that, like reverse osmosis, uses a semi-permeable membrane to effect separation of water from dissolved solutes.
The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration (relative to that of the feed solution), is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis. The simplest equation describing the relationship between osmotic and hydraulic pressures and water (solvent) flux is: where indicating reverse osmotic flow).
The solute flux ( Vapour pressure of water. The vapour pressure of water is the pressure at which water vapour is in thermodynamic equilibrium with its condensed state.
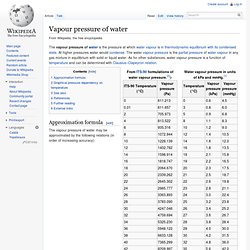
At higher pressures water would condense. The water vapour pressure is the partial pressure of water vapour in any gas mixture in equilibrium with solid or liquid water. As for other substances, water vapour pressure is a function of temperature and can be determined with Clausius–Clapeyron relation. Approximation formula[edit] Propiedades termofísicas de sistemas fluidos. Weather Forecast - México, MX - Local & Long Range. Cálculo de la constante de los gases. Humid Air and the Ideal Gas Law. Daltons Law and Pressure in Moist Air. Pressure in a mixture of dry air and water vapor - humid or moist air - can be estimated by using Daltons Law of Partial Pressures Daltons Law states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the constituent gases.

The partial pressure is the pressure each gas would exert if it alone occupied the volume of the mixture. Air - Temperature, Pressure and Density. La tecnologia de la desalacion - Lenntech. Water purification. Control room and schematics of the water purification plant to Lac de Bret, Switzerland Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from contaminated water.
The goal of this process is to produce water fit for a specific purpose. Most water is disinfected for human consumption (drinking water) but water purification may also be designed for a variety of other purposes, including meeting the requirements of medical, pharmacological, chemical and industrial applications. In general the methods used include physical processes such as filtration, sedimentation, and distillation, biological processes such as slow sand filters or biologically active carbon, chemical processes such as flocculation and chlorination and the use of electromagnetic radiation such as ultraviolet light.
The standards for drinking water quality are typically set by governments or by international standards. History Sand filter U.S. Treatment. Drinking water. Drinking water or potable water is water safe enough to be consumed by humans or used with low risk of immediate or long term harm.
In most developed countries, the water supplied to households, commerce and industry meets drinking water standards, even though only a very small proportion is actually consumed or used in food preparation. Typical uses (for other than potable purposes) include toilet flushing, washing and landscape irrigation. The word potable came into English from the Late Latin potabilis meaning drinkable. Over large parts of the world, humans have inadequate access to potable water and use sources contaminated with disease vectors, pathogens or unacceptable levels of toxins or suspended solids. Drinking or using such water in food preparation leads to widespread acute and chronic illnesses and is a major cause of death and misery in many countries. Requirements[edit] Osmotic-pressure-calculation. This calculator is under construction This calculator determines the osmotic pressure of a solution containing dissolved solids.
The osmotic pressure is based on theoretical calculations and gives you an indication about the operational pressure of a reverse osmosis system. The osmotic pressure can be calculated with the following formula: Filtros de agua y sistemas de osmosis inversa: Osmosis inversa domestica y equipos de osmosis inversa para el hogar con UV. Osmosis inversa domestica de cinco etapas con flush Manual Kits de ósmosis inversa fáciles y rápidos de instalar por el propio usuario sin herramientas especiales ni conocimientos de fontanería.
El equipo de Osmosis Inversa se instala bajo el fregadero de la cocina y viene provisto de un depósito de 12 litros de reserva de capacidad útil. ¿Como funciona la osmosis inversa? 1º El agua pasa por un prefiltro de sedimentos que elimina sólidos en suspensión (tierra, barro, arena, óxidos, etc.) con un tamaño mayor de 5 micras. Untitled. VONTRON® Residential Membrane Elements Brief Introduction The 1812-sized and 2012-sized residential membrane elements are mainly applicable to various small-sized systems, such as household water purifier and other water purifying devices in hospital and laboratory. Being suitable for the desalting treatment of those water sources with salt concentration lower than 2000 ppm, such as surface water, underground water, tap water and municipal water, etc., ULP series are mainly applicable to numerous applications of various sizes, such as pure water, boiler water replenishment, foodstuff processing, and pharmaceutical production, etc.
Specifications and Major Properties Testing Conditions: Testing Pressure…………………………………60 psi (0.41Mpa) Equipos de Filtración por Osmosis Inversa, osmosis reversa comercial y desalinadoras. EQUIPOS PARA EMBOTELLADORAS CON OSMOSIS INVERSA DE AGUA PARA BEBER. Osmosis inversa, Nano, Ultra y Micro filtración. Osmosis Inversa Purificadoras.