

Heat engine. In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical energy which can then be used to do mechanical work.[1][2] It does this by bringing a working substance from a higher state temperature to a lower state temperature.
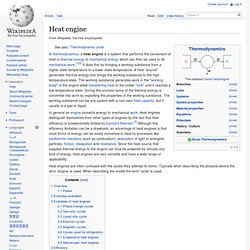
A heat "source" generates thermal energy that brings the working substance to the high temperature state. The working substance generates work in the "working body" of the engine while transferring heat to the colder "sink" until it reaches a low temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. Heat engines are often confused with the cycles they attempt to mimic. Pistonless rotary engine. A pistonless rotary engine is an internal combustion engine that does not use pistons in the way a reciprocating engine does, but instead uses one or more rotors, sometimes called rotary pistons.
An example of a pistonless rotary engine is the Wankel engine. The term rotary combustion engine has been suggested[by whom?] Timeline of heat engine technology. This Timeline of heat engine technology describes how heat engines have been known since antiquity but have been made into increasingly useful devices since the seventeenth century as a better understanding of the processes involved was gained.
They continue to be developed today. In engineering and thermodynamics, a heat engine performs the conversion of heat energy to mechanical work by exploiting the temperature gradient between a hot "source" and a cold "sink". Heat is transferred to the sink from the source, and in this process some of the heat is converted into work. Engines.