

Introduction to quantum mechanics. Many aspects of quantum mechanics are counterintuitive[3] and can seem paradoxical, because they describe behavior quite different from that seen at larger length scales.
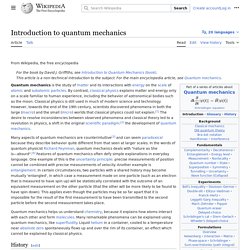
In the words of quantum physicist Richard Feynman, quantum mechanics deals with "nature as She is – absurd".[4] For example, the uncertainty principle of quantum mechanics means that the more closely one pins down one measurement (such as the position of a particle), the less accurate another measurement pertaining to the same particle (such as its momentum) must become. The first quantum theory: Max Planck and black-body radiation[edit] Hot metalwork. Uncertainty principle. Where ħ is the reduced Planck constant.
The original heuristic argument that such a limit should exist was given by Heisenberg, after whom it is sometimes named the Heisenberg principle. This ascribes the uncertainty in the measurable quantities to the jolt-like disturbance triggered by the act of observation. Though widely repeated in textbooks, this physical argument is now known to be fundamentally misleading.[4][5] While the act of measurement does lead to uncertainty, the loss of precision is less than that predicted by Heisenberg's argument; the formal mathematical result remains valid, however. Since the uncertainty principle is such a basic result in quantum mechanics, typical experiments in quantum mechanics routinely observe aspects of it. Mathematical formulation of quantum mechanics.