

Photographic Features. Physics I: Classical Mechanics. Alpha: Computational Knowledge Engine. Dive R1615 Highlights. After 32,000 Years, an Ice Age Flower Blooms Again. Big Bang Was Actually a Phase Change: New Theory. How did the universe begin?

The Big Bang is traditionally envisioned as the moment when an infinitely dense bundle of energy suddenly burst outward, expanding in three spatial directions and gradually cooling down as it did so. Now, a team of physicists says the Big Bang should be modeled as a phase change: the moment when an amorphous, formless universe analogous to liquid water cooled and suddenly crystallized to form four-dimensional space-time, analogous to ice. In the new study, lead author James Quach and colleagues at the University of Melbourne in Australia say the hypothesis can be tested by looking for defects that would have formed in the structure of space-time when the universe crystallized. The universe is currently about 13.7 billion years old. "Think of the early universe as being like a liquid," Quach said in a statement. The research by Quach and his team is detailed in this month's edition of the journal Physical Review D.
Water forms floating 'bridge' when exposed to high voltage. While it's one of the most important and abundant chemical compounds on Earth, water is still a puzzle to scientists.
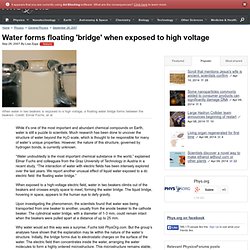
Much research has been done to uncover the structure of water beyond the H2O scale, which is thought to be responsible for many of water’s unique properties. However, the nature of this structure, governed by hydrogen bonds, is currently unknown. “Water undoubtedly is the most important chemical substance in the world,” explained Elmar Fuchs and colleagues from the Graz University of Technology in Austria in a recent study. “The interaction of water with electric fields has been intensely explored over the last years. We report another unusual effect of liquid water exposed to a dc electric field: the floating water bridge.” When exposed to a high-voltage electric field, water in two beakers climbs out of the beakers and crosses empty space to meet, forming the water bridge. Why water would act this way was a surprise, Fuchs told PhysOrg.com. Copyright 2007 PhysOrg.com. Novel chemical reaction.
(Phys.org) -- A chemical reaction reported by University of Delaware assistant professor Donald Watson and his laboratory group has set the chemistry world abuzz for its creativity and potential utility.
Watson and his team in the UD Department of Chemistry and Biochemistry have developed a chemical reaction that converts carbon-hydrogen bonds to carbon-silicon bonds using the metal palladium as a catalyst, yielding an important new tool for building molecules. The potential industrial applications are broad, ranging from the manufacture of medicines to plastics. Watson presented the research on March 25 during the national meeting of the American Chemical Society (ACS) in San Diego.
The work subsequently was highlighted in the April 2 issue of the ACS journal Chemical and Engineering News. The research was inspired, Watson says, by the work of Richard F. “Silicon is one row below carbon on the Periodic Table, so we’re activating a way to move down the table,” Watson notes.