

Leggi di Boyle e Gay Lussac. Facciamo Scienze - esperienza 22. Esperimento di chimica al liceo - istituto paritario Visconti di Roma - Combustione. Idee della chimica - Le leggi dei gas. Pressure and temperature relationship of a gas – The Pressure Law - Pass My Exams: Easy exam revision notes for GSCE Physics. Pressure and temperature relationship of a gas The Pressure Law The pressure law states: "For a fixed mass of gas, at a constant volume, the pressure (p) is directly proportional to the absolute temperature (T).
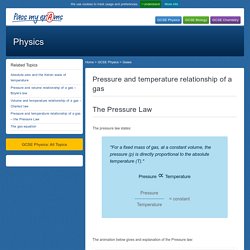
" Pressure ∝ Temperature The animation below gives and explanation of the Pressure law: A sealed cylinder with no leaks contains a fixed mass. The following set up is used to investigate the relationship between temperature and pressure for a gas. Plotting the pressure (p) against the absolute temperature (T) gives a straight line which when extrapolated passes through the origin. Pressure Law Example: Using the example of the sealed cylinder above the pressure of gas is recorded as 1.0 x 105 N/m2 at a temperature of 0°C.
Solution: We know p/T = constant therefore, p1/T1 = p2/T2 p1 = 1.0 x 105 N/m2 T1 = 0°C = 0+273 = 273K (remember to convert from Celsius to Kelvin) T2 = 150°C = 150+273 = 423K p2 =? Volume and temperature relationship of a gas – Charles' law - Pass My ExamsPass My Exams. Volume and temperature relationship of a gas Charles' law The relationship between the volume and temperature of a gas was first put forward by the French scientist Jacques-Alexandre-César Charles at around 1787 and is known as Charles’ Law.

Charles’ law states: "For a fixed mass of gas, at a constant pressure, the volume (V) is directly proportional to the absolute temperature (T). " Volume α Temperature The animation below gives and explanation of Charles' law: A sealed cylinder with no leaks contains a fixed mass. The above set up is used to investigate the relationship between temperature and volume for a gas. By plotting the recorded values of volume (V) against temperature (T) a straight line is produced. Converting the recorded temperatures into the Kelvin scale and plotting the volume (V) against the absolute temperature (T) gives a straight line which when extrapolated passes through the origin. Charles’ Law Example: Solution: We know V/T = constant therefore, Pressure and volume relationship of a gas – Boyle's law - Pass My Exams: Easy exam revision notes for GSCE Physics.
Pressure and volume relationship of a gas Boyle's law All the particles (atoms and molecules) of a substance are continually moving and so possess kinetic energy.

In gases the movement of the particles is highly energetic and this is the reason why gases form, the particles have enough energy to overcome the attractive forces holding the particles together. In gases the particles are moving very quickly and freely in a random manner constantly bumping into each other and their surroundings. It is these collisions between the particles of the gas and the walls of the container it is confined to that creates gas pressure. The relationship of a gas with pressure and volume was developed by the scientist Robert Boyle at around 1660 and is known as Boyle’s Law. Boyle’s law states: "For a fixed mass of gas, at a constant temperature, the product (pressure x volume) is a constant.
" Pressure x Volume = constant p x V = constant The animation below gives and explanation of Boyle's law: Solution: We know.