

Curie constant. The Curie constant is a material-dependent property that relates a material's magnetic susceptibility to its temperature.
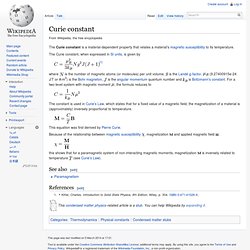
The Curie constant, when expressed in SI units, is given by where is the number of magnetic atoms (or molecules) per unit volume, is the Landé g-factor, (9.27400915e-24 J/T or A·m2) is the Bohr magneton, is the angular momentum quantum number and is Boltzmann's constant. . , the formula reduces to The constant is used in Curie's Law, which states that for a fixed value of a magnetic field, the magnetization of a material is (approximately) inversely proportional to temperature. This equation was first derived by Pierre Curie. Because of the relationship between magnetic susceptibility , magnetization and applied magnetic field this shows that for a paramagnetic system of non-interacting magnetic moments, magnetization is inversely related to temperature (see Curie's Law).
See also[edit] Paramagnetism References[edit] Magnetic susceptibility. In electromagnetism, the magnetic susceptibility Definition of volume susceptibility[edit] The volume magnetic susceptibility, represented by the symbol (often simply , sometimes – magnetic, to distinguish from the electric susceptibility), is defined in the International System of Units — in other systems there may be additional constants — by the following relationship, it is same as residual magnet. where M is the magnetization of the material (the magnetic dipole moment per unit volume), measured in amperes per meter, and H is the magnetic field strength, also measured in amperes per meter.
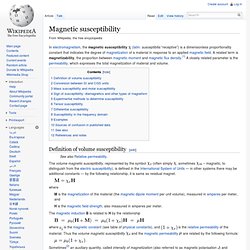
The magnetic induction B is related to H by the relationship where μ0 is the magnetic constant (see table of physical constants), and and the magnetic permeability are related by the following formula: This allows an alternative description of all magnetization phenomena in terms of the quantities I and B, as opposed to the commonly used M and H. Conversion between SI and CGS units[edit] Examples[edit] Boltzmann constant. The Boltzmann constant (kB or k), named after Ludwig Boltzmann, is a physical constant relating energy at the individual particle level with temperature.
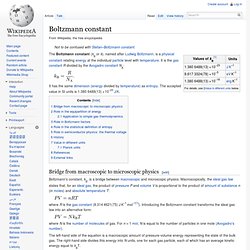
It is the gas constant R divided by the Avogadro constant NA: It has the same dimension (energy divided by temperature) as entropy. The accepted value in SI units is 1.3806488(13)×10−23 J/K. Bridge from macroscopic to microscopic physics[edit] where R is the gas constant (8.314 4621(75) J K−1 mol−1[1]). The left-hand side of the equation is a macroscopic amount of pressure-volume energy representing the state of the bulk gas. Role in the equipartition of energy[edit] Given a thermodynamic system at an absolute temperature T, the thermal energy carried by each microscopic "degree of freedom" in the system is on the order of magnitude of kBT/2 (i. e., about 2.07×10−21 J, or 0.013 eV, at room temperature).
Application to simple gas thermodynamics[edit] Kinetic theory gives the average pressure p for an ideal gas as gives History[edit] Bohr magneton. In atomic physics, the Bohr magneton (symbol μB) is a physical constant and the natural unit for expressing an electron magnetic dipole moment.
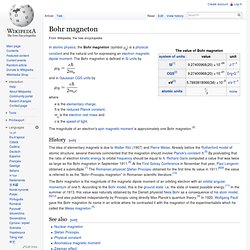
The Bohr magneton is defined in SI units by and in Gaussian CGS units by where e is the elementary charge, ħ is the reduced Planck constant, me is the electron rest mass and c is the speed of light. The magnitude of an electron's spin magnetic moment is approximately one Bohr magneton.[4] History[edit] The idea of elementary magnets is due to Walter Ritz (1907) and Pierre Weiss. The Bohr magneton is the magnitude of the magnetic dipole moment of an orbiting electron with an orbital angular momentum of one ħ. See also[edit] References[edit] Jump up ^ "CODATA value: Bohr magneton".