

0. Alkaloid. The first individual alkaloid, morphine, was isolated in 1804 from poppy (Papaver somniferum).[1] The boundary between alkaloids and other nitrogen-containing natural compounds is not clear-cut.[8] Compounds like amino acid peptides, proteins, nucleotides, nucleic acid, amines, and antibiotics are usually not called alkaloids.[2] Natural compounds containing nitrogen in the exocyclic position (mescaline, serotonin, dopamine, etc.) are usually attributed to amines rather than alkaloids.[9] Some authors, however, consider alkaloids a special case of amines.[10][11][12] Naming[edit] The article that introduced the concept of "alkaloid".
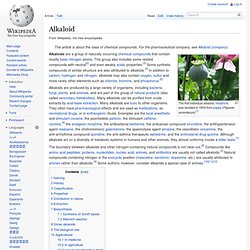
The name "alkaloids" (German: Alkaloide) was introduced in 1819 by the German chemist Carl Friedrich Wilhelm Meißner, and is derived from late Latin root Latin: alkali (which, in turn, comes from the Arabic al-qalwī – "ashes of plants") and the suffix Greek: -οειδής – "like". History[edit] Classification[edit] Properties[edit] Distribution in nature[edit] 1. "True alkaloids" 1.1 "True alkaloids" 1.1.1 Acridine. Acridine, C13H9N, is an organic compound and a nitrogen heterocycle.
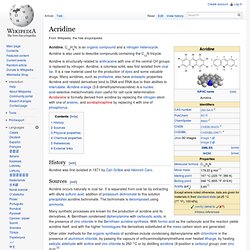
Acridine is also used to describe compounds containing the C13N tricycle. History[edit] Acridine was first isolated in 1871 by Carl Gräbe and Heinrich Caro. 1.1.2 Imidazole. Imidazole is an organic compound with the formula (CH)2N(NH)CH.
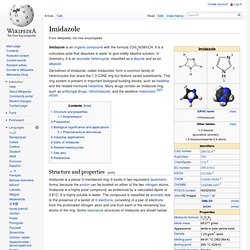
It is a colourless solid that dissolves in water to give mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole and as an alkaloid. Derivatives of imidazole, called imidazoles, form a common family of heterocycles that share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building-blocks, such as histidine, and the related hormone histamine. Many drugs contain an imidazole ring, such as antifungal drugs, nitroimidazole, and the sedative midazolam.[2][3][4][5][6] Structure and properties[edit] 1.1.3 Indole. Indole is an aromatic heterocyclic organic compound.
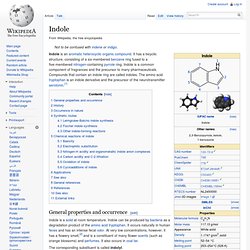
It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a common component of fragrances and the precursor to many pharmaceuticals. 1.1.3.1 Isoprenoid. 1.1.3.1.1 Ergot. 1.1.3.1.1.1 Ergotamine. Ergotamine is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It possesses structural similarity to several neurotransmitters, and has biological activity as a vasoconstrictor. It is used medicinally for treatment of acute migraine attacks (sometimes in combination with caffeine). Medicinal usage of ergot fungus began in the 16th century to induce childbirth, yet dosage uncertainties discouraged the use.
It has been used to prevent post-partum haemorrhage (bleeding after childbirth). It was first isolated from the ergot fungus by Arthur Stoll at Sandoz in 1918 and marketed as Gynergen in 1921.[4] Mechanism of action[edit] Biosynthesis[edit] Drug uses[edit] 1.1.3.1.1.2 Ergotoxine. 1.1.3.1.1.3 Ergoxine. 1.1.3.1.1.4 Water-Insoluble Polypeptide Derivatives. 1.1.3.1.1.5 Water-Soluble Aminoalcohol Derivatives. 1.1.3.1.2 Monoterpenoids. Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16.
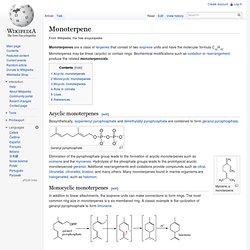
Monoterpenes may be linear (acyclic) or contain rings. Biochemical modifications such as oxidation or rearrangement produce the related monoterpenoids. Acyclic monoterpenes[edit] Biosynthetically, isopentenyl pyrophosphate and dimethylallyl pyrophosphate are combined to form geranyl pyrophosphate. Geranyl pyrophosphate Myrcene, a monoterpene Elimination of the pyrophosphate group leads to the formation of acyclic monoterpenes such as ocimene and the myrcenes. Monocyclic monoterpenes[edit] In addition to linear attachments, the isoprene units can make connections to form rings. The terpinenes, phellandrenes, and terpinolene are formed similarly. Bicyclic monoterpenes[edit] Geranyl pyrophosphate can also undergo two sequential cyclization reactions to form bicyclic monoterpenes, such as pinene which is the primary constituent of pine resin. Role in climate[edit] 1.1.3.1.2.1 Acyclic. 1.1.3.1.2.2 Bicyclic.
1.1.3.1.2.3 Monocyclic. 1.1.3.1.3 Bisindole. 1.1.3.1.3.1 Calicantine. 1.1.3.1.3.2 Toxiferine. 1.1.3.1.3.3 Villalstonine. The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile[1][2][3] to an α,β-unsaturated carbonyl compound.
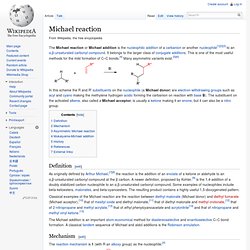
It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds.[4] Many asymmetric variants exist.[5][6] Definition[edit] As originally defined by Arthur Michael,[7][8] the reaction is the addition of an enolate of a ketone or aldehyde to an α,β-unsaturated carbonyl compound at the β carbon. A newer definition, proposed by Kohler,[9] is the 1,4-addition of a doubly stabilized carbon nucleophile to an α,β-unsaturated carbonyl compound.
The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation. 1.1.3.1.3.4 Vinblastine + Vincristine. 1.1.3.1.3.5 Voacamine. The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia.
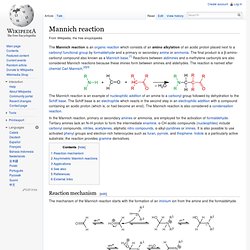
The final product is a β-amino-carbonyl compound also known as a Mannich base.[1] Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after chemist Carl Mannich.[2][3] The Mannich reaction is an example of nucleophilic addition of an amine to a carbonyl group followed by dehydration to the Schiff base. The Schiff base is an electrophile which reacts in the second step in an electrophilic addition with a compound containing an acidic proton (which is, or had become an enol).
The Mannich reaction is also considered a condensation reaction. In the Mannich reaction, primary or secondary amines or ammonia, are employed for the activation of formaldehyde. 1.1.3.2 Non-isoprenoid. 1.1.3.2.1 Simple Indole Derivatives. 1.1.3.2.2 Simple Derivatives of β-carboline. Β-Carboline (9H-pyrido[3,4-b]indole) also known as norharmane is a nitrogen containing heterocycle.
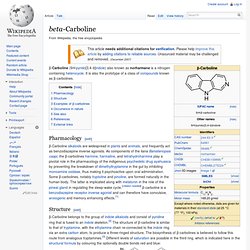
It is also the prototype of a class of compounds known as β-carbolines. Pharmacology[edit] β-Carboline alkaloids are widespread in plants and animals, and frequently act as benzodiazepine inverse agonists. As components of the liana Banisteriopsis caapi, the β-carbolines harmine, harmaline, and tetrahydroharmine play a pivotal role in the pharmacology of the indigenous psychedelic drug ayahuasca by preventing the breakdown of dimethyltryptamine in the gut by inhibiting monoamine oxidase, thus making it psychoactive upon oral administration. Some β-carbolines, notably tryptoline and pinoline, are formed naturally in the human body. Structure[edit] Examples of β-carbolines[edit] Some of the more important β-carbolines are tabulated by structure below.
Occurrence in nature[edit] Eight plant families are known to express 64 different kinds of β-carboline alkaloids. 1.1.3.2.3 Pyrolo-Indole. 1.1.4 Indolizidine. 1.1.5 Isoquinoline. Isoquinoline is a heterocyclic aromatic organic compound.
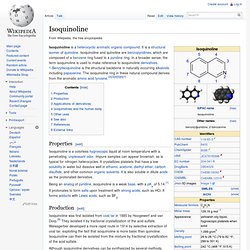
It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.[2][3][4][5][6][7] 1.1.6 Isoxazole. Isoxazole is an azole with an oxygen atom next to the nitrogen.
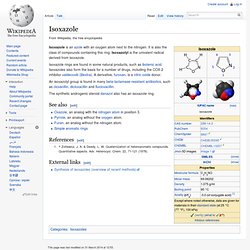
It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole. Isoxazole rings are found in some natural products, such as ibotenic acid. Isoxazoles also form the basis for a number of drugs, including the COX-2 inhibitor valdecoxib (Bextra). A derivative, furoxan, is a nitric oxide donor. An isoxazolyl group is found in many beta-lactamase-resistant antibiotics, such as cloxacillin, dicloxacillin and flucloxacillin. 1.2 "True alkaloids" 1.2.7 Oxazole. Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds.
These are azoles with an oxygen and a nitrogen separated by one carbon.[2] Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole. Preparation[edit] Classical oxazole synthetic methods in organic chemistry are Other methods are reported in literature. Oxazolines can also be obtained from cycloisomerization of certain propargyl amides. In one reported oxazole synthesis the reactants are a nitro-substituted benzoyl chloride and an isonitrile:[4] [5] 1.2.8 Piperidine. Production[edit] Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst:[4] Pyridine can also be reduced to piperidine via a modified Birch reduction using sodium in ethanol.[5] Natural occurrence of piperidine and derivatives[edit] Piperidine itself has been obtained from black pepper,[6] from Psilocaulon absimile N.E.Br (Aizoaceae),[7] and in Petrosimonia monandra.[8] The piperidine structural motif is present in numerous natural alkaloids.
Anaferin. 1.2.9 Pyridine. 1.2.10 Pyrrolidine. Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that is ammoniacal, fishy, shellfish-like.[4] Compared to acyclic secondary amines, it is about 10 times more basic. Synthesis and occurrence[edit] Pyrrolidine is produced industrially by treatment of 1,4-butanediol with ammonia over an oxide catalyst.[5] Pyrrolidine is found in the leaves of tobacco and carrot.
Nicotine contains an N-methylpyrrolidine ring linked to a pyridine ring. 1.2.11 Pyrrolizidine. 1.2.12 Purine. A purine is a heterocyclic aromatic organic compound. It consists of a pyrimidine ring fused to an imidazole ring. Purines, which include substituted purines and their tautomers, are the most widely occurring nitrogen-containing heterocycle in nature.[1] Purines and pyrimidines make up the two groups of nitrogenous bases, including the two groups of nucleotide bases.
Two of the four deoxyribonucleotides and two of the four ribonucleotides, the respective building-blocks of DNA and RNA, are purines. Notable purines[edit] Other notable purines are hypoxanthine (4), xanthine (5), theobromine (6), caffeine (7), uric acid (8) and isoguanine (9). 1.3 "True alkaloids" 1.3.13 Quinazoline. Quinazoline is a heterocyclic compound made up of two fused six-membered simple aromatic rings, a benzene ring and a pyrimidine ring. 1.3.14 Quinoline. Occurrence and isolation[edit] Quinoline was first extracted from coal tar in 1834 by Friedlieb Ferdinand Runge.[3] Coal tar remains the principal source of commercial quinoline.[4] Like other nitrogen heterocyclic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites. 1.3.15 Quinolizidine.
Quinolizidine (norlupinane, octahydro-2H-quinolizine) is a nitrogen-containing heterocyclic compound. 1.3.16 Thiazole. Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS.[2] The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure[edit] Thiazoles are members of the azoles heterocycles that includes imidazoles and oxazoles.
1.3.17 Tropane. The nitrogen bridge is between C-1 and C-5; there are two asymmetric carbons, but tropane is optically inactive due to symmetry. 2. Protoalkaloids. 2.1 Benzylamine. Benzylamine is the chemical compound with the formula C6H5CH2NH2. 2.2 Colchicine. Colchicine is a medication that treats gout. It is a toxic natural product and secondary metabolite, originally extracted from plants of the genus Colchicum (autumn crocus, Colchicum autumnale, also known as "meadow saffron"). 2.3 Muscarine. Amanita muscaria Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata.
2.4 Phenethylamine. 3. Polyamine alkaloids. 3.1 Putrescine. Putrescine, or tetramethylenediamine, is a foul-smelling[2] organic chemical compound NH2(CH2)4NH2 (1,4-diaminobutane or butanediamine) that is related to cadaverine; both are produced by the breakdown of amino acids in living and dead organisms and both are toxic in large doses.[3][4] The two compounds are largely responsible for the foul odor of putrefying flesh, but also contribute to the odor of such processes as bad breath and bacterial vaginosis.[5] They are also found in semen and some microalgae, together with related molecules like spermine and spermidine.
History[edit] Putrescine[6] and cadaverine[7] were first described in 1885 by the Berlin physician Ludwig Brieger (1849–1919).[8] 3.2 Spermidine. 3.3 Spermine. 4. Peptide Alkaloids. 4.1 Peptide alkaloids with a 13-membered cycle. 4.2 Peptide alkaloids with a 14-membered cycle. 4.3 Peptide alkaloids with a 15-membered cycle. 5. Pseudalkaloids. 5.1 Steroid. 5.2 Terpene.