

2-Arachidonoylglycerol. 2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor.[1][2] It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol.
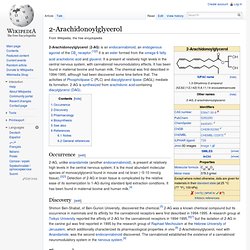
It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994-1995, although had been discovered some time before that. The activities of Phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).
Occurrence[edit] Discovery[edit] Shimon Ben-Shabat, of Ben-Gurion University, discovered the chemical.[5] 2-AG was a known chemical compound but its occurrence in mammals and its affinity for the cannabinoid receptors were first described in 1994-1995. Anandamide. §History[edit] Anandamide was isolated and its structure first described in 1992 by W.
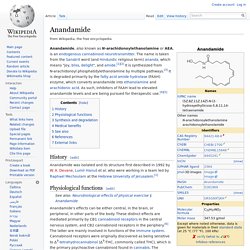
A. Devane, Lumír Hanuš et al. who were working in a team led by Raphael Mechoulam at the Hebrew University of Jerusalem.[1] §Physiological functions[edit] Anandamide has been shown to impair working memory in rats.[7] Studies are under way to explore what role anandamide plays in human behavior, such as eating and sleep patterns, and pain relief. Anandamide is also important for implantation of the early stage embryo in its blastocyst form into the uterus.
Anandamide plays a role in the regulation of feeding behavior, and the neural generation of motivation and pleasure. A study published in 1998 shows that anandamide inhibits human breast cancer cell proliferation.[12] Some studies have linked anandamide release as a mechanism of analgesic effects induced by exercise, particularly by running.[13] In 1996, researchers discovered anandamide in chocolate. §Synthesis and degradation[edit] §See also[edit] Arachidonic acid. Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6).
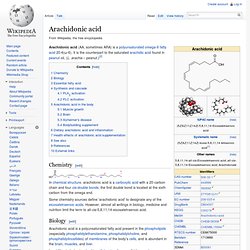
It is the counterpart to the saturated arachidic acid found in peanut oil, (L. arachis – peanut.)[2] Chemistry[edit] In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four cis-double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designate any of the eicosatetraenoic acids. However, almost all writings in biology, medicine and nutrition limit the term to all-cis-5,8,11,14-eicosatetraenoic acid. Biology[edit] In addition to being involved in cellular signaling as a lipid second messenger involved in the regulation of signaling enzymes, such as PLC-γ, PLC-δ, and PKC-α, -β, and -γ isoforms, arachidonic acid is a key inflammatory intermediate and can also act as a vasodilator.[3] (Note separate synthetic pathways, as described in section below.)
Essential fatty acid[edit]