

Lithium Battery Research: Lithium Battery life, durability, and failure. Faraday efficiency. Sources of faradaic loss[edit] Faradaic losses are experienced by both electrolytic and galvanic cells when electrons or ions participate in unwanted side reactions.
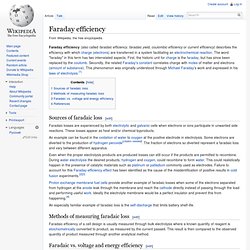
These losses appear as heat and/or chemical byproducts. An example can be found in the oxidation of water to oxygen at the positive electrode in electrolysis. Some electrons are diverted to the production of hydrogen peroxide[citation needed]. The fraction of electrons so diverted represent a faradaic loss and vary between different apparatus. Even when the proper electrolysis products are produced losses can still occur if the products are permitted to recombine.
Russian Journal of Electrochemistry, Volume 36, Number 7. Applied Physics A: Materials Science & Processing, Volume 49, Number 1. Li Ion Conducting Polymer Gel Electrolytes Based on Ionic Liquid/PVDF-HFP Blends. Solid polymer electrolyte lithium. This promising lithium rechargeable technology is using a polymer electrolyte in a solid state cell in which a polymer electrolyte is sandwiched between a lithium metal film and a metal film.

By dissolving lithium, not into a liquid electrolyte but into a really thin polymer (plastic), a high-power battery is realized that is light, yet durable. The laminate construction of such cells offer flexibility of shape and size, which is advantageous for portable power source applications. However, at the present time, the conductivity of these batteries is very low at room temperature, compared with those of liquid electrolytes: these batteries are normally operated in the 60-120oC range. Research is being aimed at increasing conductivity through the use of plasticizers and new polymers. This new technology offers the potential of future low manufacturing costs. This unique battery contains a solid, dry, polymer electrolyte with a metallic lithium anode.
See other electrochemical cells. Iron-air batteries may prove a cheap, eco-friendly solution for energy storage. Revamping a concept that was first explored forty years ago, researchers at the University of Southern California (USC) are putting the final touches on a patent-pending design for cheap, rechargeable, high energy density iron-air batteries.
Because of their unique features, the batteries look particularly well-suited to the kind of large-scale energy storage that could accelerate the adoption of renewable energy sources. The quest for a cheap, environmentally friendly rechargeable battery stretches back for decades. For one, lithium-ion batteries were first proposed in the seventies, and only recent advances in materials technology have made this technology into one of the most common, high-performing solutions for today's portable electronics. Now, a team of USC researchers may have found the key to resuscitating yet another design first proposed around the same time – the iron-air battery. Make an Aluminum Air Battery. Theoretical Energy Density of Li–Air Batteries. + Author Affiliations Abstract A model for predication of the gravimetric and volumetric energy densities of Li-air batteries using aqueous electrolytes is developed.

The theoretical gravimetric/volumetric capacities and energy densities are calculated based on the minimum weight of the electrolyte and volume of air electrode needed for completion of the electrochemical reaction with Li metal as an anode electrode. It was determined that both theoretical gravimetric/volumetric capacities and energy densities are dependent on the porosity of the air electrode. For instance, at a porosity of 70%, the maximum theoretical cell capacities are and in basic electrolyte, and and in acidic electrolyte. Key Words Footnotes ↵ *Electrochemical Society Active Member Manuscript received February 8, 2008.
Mamma.com. Review on Li-air batteries - Opportunities, limitations and perspective. Lithium−Air Battery: Promise and Challenges - The Journal of Physical Chemistry Letters. IBM Research - Almaden, 650 Harry Road, San José, California 95120 J.
Phys. Chem. Lett., 2010, 1 (14), pp 2193–2203 *To whom correspondence should be addressed. Biography Dr. Dr. Dr. Lithium-Air Batteries: An Overview. Yuan Zhong December 3, 2011 Submitted as coursework for PH240, Stanford University, Fall 2011 Introduction The technological revolution over the past centuries consumes vast amount of energy and results in significant carbon footprint.

A dominate proportion of overall energy demand is attributed to transportation sector which leads to various environmental problems, such as urban air pollution. Oxygen Selective Membranes for Li-Air (O2) Batteries. Cathodes for Solid-State Lithium-Oxygen Cells: Roles of Nasicon Glass-Ceramics. Lithium–air battery. The major appeal of the Li-air battery is the extremely high energy density, a measure of the amount of energy a battery can store for a given mass.
A lithium-air battery has an energy density (per kilo) comparable to traditional gasoline per kilo. Li-air batteries gain this advantage in energy density since they use oxygen from the air instead of storing an oxidizer internally. The technology requires significant research in a variety of fields before a viable commercial implementation is expected.[1] Four approaches are being pursued; aprotic,[2][3] aqueous,[4] solid state,[5] and mixed aqueous/aprotic.[6] Lithium batteries have received considerable attention over the past 40 years. The first commercial lithium cells arrived 20 years ago. MechE - Yang Shao-Horn.
Gail E.

Kendall Professor of Mechanical EngineeringElectrochemical Energy Laboratory, 31-056 Room 3-334Massachusetts Institute of Technology77 Massachusetts AvenueCambridge MA 02139-4307Phone: 617-253-2259 Fax: 617-258-7018 Email: shaohorn@mit.eduWeb: Curriculum Vitae Administrative Contact:Marjorie A. Inassignee:"Moltech Corporation" Electricity Storage - Metal-Air Batteries.
Transition metal cost. A New Class of Electrocatalysts for Hydrogen Production from Water Electrolysis: Metal Monolayers Supported on Low-Cost Transition Metal Carbides - Journal of the American Chemical Society. Department of Chemical Engineering, University of Delaware, Newark, Delaware 19716, United States J.
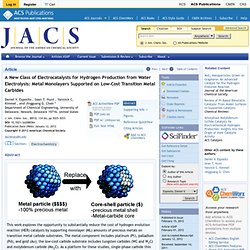
Am. Chem. Soc., 2012, 134 (6), pp 3025–3033 DOI: 10.1021/ja208656v Publication Date (Web): January 12, 2012 Copyright © 2012 American Chemical Society. Abundance of the chemical elements. Estimated proportions of matter, dark matter and dark energy in the universe.
Only the fraction of the mass and energy in the universe labeled "atoms" is composed of chemical elements. Reduce "over potentials" of the oxygen reduction reaction. Chemical Reviews.pdf (application/pdf Object) Mamma.com. Advanced materials for sodium-beta alumina batteries: Status, challenges and perspectives. Journal of Power Sources 195 (2010) 2431–2442 Contents lists available at ScienceDirect Journal of Power Sources journal homepage: www.elsevier.com/locate/jpowsour. Single solid oxide fuel cell modeling and optimization. Mamma.com. Batteries that Breathe. Rechargeable Li–O2 batteries with a covalently coupled MnCo2O4–graphene hybrid as an oxygen cathode catalyst - Energy & Environmental Science. Rechargeable Fuel Cell With Double Cathode. 8021792. Mamma.com. Polyoxometalates: from inorganic chemistry to materials science. Page 1 [Frontiers in Bioscience 9, 1759-1770, May 1, 2004]1759POLYOXOMETALATES: FROM INORGANIC CHEMISTRY TO MATERIALS SCIENCENieves Casañ-Pastor and Pedro Gómez-RomeroInstitut de Ciència de Materials de Barcelona, CSIC, Campus UAB 08193 Bellaterra, Barcelona, SpainTABLE OF CONTENTS1.
Abstract2. Introduction3. Polyoxometalates as materials3.1. Supported Polyoxometalates and Modified Surfaces4. Page 2 Polyoxometalates: from inorganic chemistry to materials science1760and condensation reactions (1, 2), driven by the veryrich acid–base chemistry of some transition metalcations, primarily W, Mo, and V. Mamma.com.
Grid Energy Power Storage. To the uninitiated, it may seem that grid energy storage is a non-sequitur, since supply often barely meets demand. However, peak period shortfalls are precisely the reason grid power storage is so critical to a consistent power supply. Grid storage systems would preserve those kilowatts that would otherwise go to waste during low demand periods. This ability is key to conserving energy and keeping the cost of electricity at an affordable level. However, to date, no single grid energy storage alternative has earned widespread approval as both cost-effective and efficient on a large scale, although grid storage battery development is rapidly escalating, with vanadium redox flow battery technology outpacing the others.
Mamma.com. Webinar: China Grid-Scale Energy Storage: Technologies & Markets, 2012-2016 : Greentech Media. In 2012, China’s electric grid will become the largest in the world in terms of both installed generation capacity and electricity produced. China also possesses the world’s largest installed wind power base and the world’s largest declared investment in renewable energy.
These facts alone suggest that China is also the most attractive market for energy storage in the world, even though China currently has just 4% of the worldwide energy storage capacity. Whereas other markets have focused on power quality and ancillary services, China’s grid energy storage market has developed with a focus on renewable energy integration, load-shifting and peak shaving. Azure International and GTM Research forecast that pumped hydro storage capacity will double or triple by 2016 to reach 40-60 GW, while other storage technologies will rise from currently insignificant levels to over 700 MW installed by 2016.