

Quantum Number. A Google image search on the term "atom" will produce two general types of picture: Unfortunately, both of these conventional representations are incorrect (in several ways), and the electronic structure of atoms needs to be understood in terms of the Schrödinger wave equation.
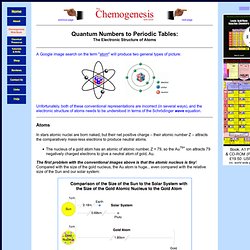
Atoms In stars atomic nuclei are born naked, but their net positive charge – their atomic number Z – attracts the comparatively mass-less electrons to produce neutral atoms. Quantum Theory of Matter. Wave-like Behavior of Matter In 1925, Louis DeBroglie hypothesized that if light, which everyone thought for so long was a wave, is a particle, then perhaps particles like the electron, proton, and neutron might have wave-like behaviors.
He went further and reasoned that since waves are described by their wavelength λ and particles are described by their momentum, p then we can relate these two variables by recalling that the Quantum Theory says and the theory of Relativity says Then let's equate these two equations to get the DeBroglie relationship between momentum (a particle property) and wavelength (a wave property) The first real experimental proof of this relationship came from Davisson and Germer in 1925, who found that electrons will diffract and interfere like waves, just like X-ray photons (light).
So, matter and light are composed of particles that have wave-like properties. Quantized States nodes are where the wave function changes from positive to negative (ends don't count!). Schrodinger equation. Review of Modern Physics. Companion Notes: Electrons in atoms. A quantum paintbox. Depending on their size, quantum dots come in many different colours, says Jonathan Cox.
The space between the stars can be quite dusty. In 1980, astronomers noticed that the starlight reflected from one particular dust cloud, or nebula, in our own galaxy contained a broad red component. For some time, the nature of the fluorescent dust particles in this nebula (the charismatic Red Rectangle), and from other areas in the Milky Way, remained a mystery.
None of the likely candidates that astronomers tested in the laboratory (including buckminsterfullerene) had optical properties that quite matched up. Then, quite unexpectedly, two groups, one European, one American, made a suggestion that did seem to fit. So what exactly is a quantum dot? A quantum dot has one fundamental, defining, and very odd, feature: its optical behaviour depends on its size. The one thing that has stopped quantum dots being used in biological experiments is their water solubility. Source: Chemistry in Britain 1. 1. Gold. The lure of gold has been the downfall of many, from those worshipping the biblical golden calf to those unsuccessfully staking their claims during the 19th century gold rushes.

Nevertheless, this lustrous metal continues to connote the pinnacle of achievement, as evidenced by Nobel Prize medals, Olympic medals, and Academy Award statuettes. Would our fascination with gold be lessened if we knew that its shiny allure was the result of excited electrons? Silver, iron, platinum, gold, and copper are all metals, which generally are malleable and ductile, conduct electricity and heat, and have a metallic luster. Some of their properties can be attributed to the way electrons are arranged in the material. In this hypothesis, each metal atom contributes its outer electrons to a shared pool. Ch 9. Unit 9 Electron Clouds and Probability The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).

This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables. ELECTRON CLOUDS AND PROBABlLlTY In the attempts to refine our model of the atom, we see the division between matter and energy has become less clear. Radiant energy is found to have many properties of particles. Small particles of matter are found to display the characteristics of wave motion. The purpose of this chapter is to look more closely at this wave-particle problem. We have seen in Chapter 8 that the frequencies predicted by Bohr for the hydrogen spectrum are "essentially" correct. Improved equipment has shown that the hydrogen spectrum lines predicted by Bohr are not single lines. WEEK 3: CHEMISTRY 111 – Chapter 9 – Electron Structure. Electromagnetic Radiation is energy and light.

We will learn how to use it to explore atomic structure. Bohr’s Theory of atomic structure is the beginning of quantum theory and describes electrons as residing in very particular "quantized" energy levels within the atom. Shells, Sub-shells and Orbitals are the backbone of the organizational structure of electrons in the atom Electron Configurations use shells, sub-shells and orbitals to describe the relative electronic organization within the atom. Periodicity of electron configurations relate to periodicity of physical and chemical properties. of valence shell configurations relate directly to relative reactivity. We know that an element is defined by the number of protons it contains. What is it, why is it important to our discussion about atoms and the arrangement of their electrons? Electromagnetic radiation is energy – we describe it as a wave – visible light is only a small portion of the electromagnetic spectrum.
Bohr’s Theory: