

Entheogen. A group of peyotes, in cultivation.
Peyote has been used in ritual contexts for thousands of years.[1][2][3] With the advent of organic chemistry, there now exist many synthetic drugs with similar psychoactive properties, many derived from these plants. Many pure active compounds with psychoactive properties have been isolated from these organisms and chemically synthesized, including mescaline, psilocybin, DMT, salvinorin A, ibogaine, ergine, and muscimol, respectively.
Semi-synthetic (e.g. LSD used by the New American Church) and synthetic drugs (e.g. Etymology[edit] The neologism entheogen was coined in 1979 by a group of ethnobotanists and scholars of mythology (Carl A. Entheogen was coined as a replacement for the terms hallucinogen and psychedelic. Ruck et al. argued that the term hallucinogen was inappropriate owing to its etymological relationship to words relating to delirium and insanity. Psilocybin. Psilocybin[nb 1] (/ˌsɪləˈsaɪbɪn/ SIL-ə-SY-bin) is a naturally occurring psychedelic compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms.
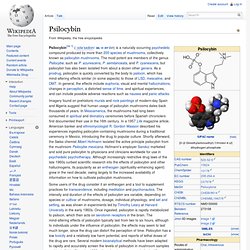
The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. Psilocin. Psilocin (also known as 4-OH-DMT, psilocine, psilocyn, or psilotsin), is a substituted tryptamine alkaloid and a serotonergic psychedelic substance.

It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances.[2] The mind-altering effects of psilocin are highly variable and subjective and resemble those of LSD and DMT. Cannabinoid. Synthetic cannabinoids encompass a variety of distinct chemical classes: the classical cannabinoids structurally related to THC, the nonclassical cannabinoids (cannabimimetics) including the aminoalkylindoles, 1,5-diarylpyrazoles, quinolines, and arylsulphonamides, as well as eicosanoids related to the endocannabinoids.[2] Cannabinoid receptors[edit] Cannabinoid receptor type 1[edit] CB1 receptors are found primarily in the brain, more specifically in the basal ganglia and in the limbic system, including the hippocampus.[1] They are also found in the cerebellum and in both male and female reproductive systems.

CB1 receptors are absent in the medulla oblongata, the part of the brain stem responsible for respiratory and cardiovascular functions. Thus, there is not the risk of respiratory or cardiovascular failure that can be produced by some drugs. Cannabinoid receptor type 2[edit] Tetrahydrocannabinol. Tetrahydrocannabinol (THC), or more precisely its main isomer (−)-trans-Δ9-tetrahydrocannabinol ( (6aR,10aR)-delta-9-tetrahydrocannabinol), is the principal psychoactive constituent (or cannabinoid) of the cannabis plant.
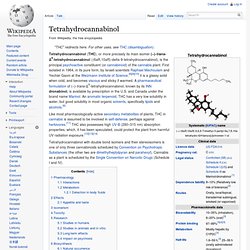
First isolated in 1964, in its pure form, by Israeli scientists Raphael Mechoulam and Yechiel Gaoni at the Weizmann Institute of Science,[8][9][10] it is a glassy solid when cold, and becomes viscous and sticky if warmed. A pharmaceutical formulation of (−)-trans-Δ9-tetrahydrocannabinol, known by its INN dronabinol, is available by prescription in the U.S. and Canada under the brand name Marinol. An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents, specifically lipids and alcohols.[6] Tetrahydrocannabinol with double bond isomers and their stereoisomers is one of only three cannabinoids scheduled by Convention on Psychotropic Substances (the other two are dimethylheptylpyran and parahexyl). Dimethyltryptamin. Vorlage:Infobox Chemikalie/Summenformelsuche vorhanden N,N-Dimethyltryptamin, kurz DMT, ist ein halluzinogenes Tryptamin-Alkaloid, welches in etlichen Pflanzen, in den Hautdrüsensekreten der Aga-Kröte sowie auch in Spuren im Menschen und in Säugetieren zu finden ist.[8][9][10] Es findet auch Anwendung als Halluzinogen bzw.
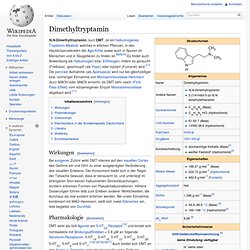
Entheogen, indem es geraucht (Freibase), geschnupft (als Yopo) oder injiziert (Fumarat) wird.[11] Die perorale Aufnahme (als Ayahuasca) wird nur bei gleichzeitiger bzw. vorheriger Einnahme von Monoaminooxidase-Hemmern (kurz MAOH oder MAOI) erreicht, da DMT sehr rasch (First-Pass-Effekt) vom körpereigenen Enzym Monoaminooxidase abgebaut wird.[11] Wirkungen[Bearbeiten] Bei exogener Zufuhr wirkt DMT intensiv auf den visuellen Cortex des Gehirns ein und führt zu einer ausgeprägten Veränderung des visuellen Erlebens.
Pharmakologie[Bearbeiten] Vorkommen[Bearbeiten] DMT ist der Hauptwirkstoff von Ayahuasca, einem kultisch verwendeten Gebräu indigener Kulturen Südamerikas. DMT.