

Christmas Cracker Chemistry. Chances are you’ll be making use of christmas crackers over the holiday period.

Chances also are that you’ve never really given a lot of thought to the chemical compounds contained therein, or the chemical reaction that makes christmas crackers go bang. Well, allow me to elaborate. Christmas crackers owe their crack to a compound called silver fulminate. This compound has the molecular formula AgCNO, and can be prepared relatively simply by reacting concentrated nitric acid with silver and ethanol. Fulminates contain the fulminate ion, CNO-, the instability of which leads to fulminate salts being friction sensitive explosives. ‘Poisonous’ Poinsettia pH Indicators. I’m making pH indicator paper with some of my classes this week, using the coloured leaves of red poinsettia plants, which set me thinking about the chemistry behind why these plants can be used as indicators.
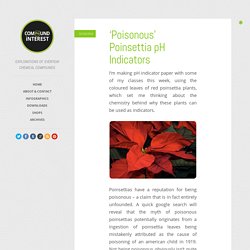
Poinsettias have a reputation for being poisonous – a claim that is in fact entirely unfounded. A quick google search will reveal that the myth of poisonous poinsettias potentially originates from a ingestion of poinsettia leaves being mistakenly attributed as the cause of poisoning of an american child in 1919. Not being poisonous obviously isn’t quite the same as being edible, and eating poinsettia leaves can potentially cause stomach pain and vomiting – but there have been no recorded deaths as a result of the plant.
As its leaves also have a reportedly ‘indescribably awful’ taste, few could probably bear more than a nibble. As any secondary school science teacher will know, it’s not just poinsettias that can be used to fashion rudimentary indicator solutions.