

Franklin's X-ray diffraction, explanation of X-ray pattern. Home DNA Learning Center Preparing students and families to thrive in the gene age Website Search Franklin's X-ray diffraction, explanation of X-ray pattern.
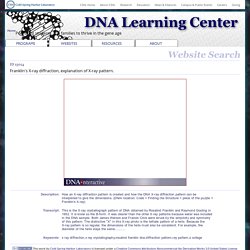
Description: How an X-ray diffraction pattern is created and how the DNA X-ray diffraction pattern can be interpreted to give the dimensions. Transcript: This is the X-ray crystallograph pattern of DNA obtained by Rosalind Franklin and Raymond Gosling in 1952. Keywords: x ray diffraction,x ray crystallography,rosalind franklin dna,diffraction pattern,ray pattern,s college This work by Cold Spring Harbor Laboratory is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 United States License. Related content: 15013. How to obtained an X-ray diffraction pattern. SOURCE: DNAi 15874. Rosalind Franklin and Raymond Gosling obtained this X-ray diffraction pattern, which triggered the idea that DNA was a helix. 15875. 15337.
Crystallography: An Animated adventure. The effect of temperature on rates of reaction. You can mark the position of activation energy on a Maxwell-Boltzmann distribution to get a diagram like this: Only those particles represented by the area to the right of the activation energy will react when they collide.

The great majority don't have enough energy, and will simply bounce apart. To speed up the reaction, you need to increase the number of the very energetic particles - those with energies equal to or greater than the activation energy. Increasing the temperature has exactly that effect - it changes the shape of the graph. In the next diagram, the graph labelled T is at the original temperature. If you now mark the position of the activation energy, you can see that although the curve hasn't moved very much overall, there has been such a large increase in the number of the very energetic particles that many more now collide with enough energy to react. Chemguide: helping you to understand Chemistry - Main Menu. IB Chemistry revision notes and syllabus. Assessment in the Diploma Program (2016) Assessment is by means of examination and practical for both higher and standard level chemistry courses.
Examination weighting - 80% Internal assessment weighting - 20% Examination (higher level) There are three papers: Paper 1 (multiple choice) - 20% Paper 2 (structured questions) - 36% Paper 3 (data and options) - 24% Total - 80% Paper 1 (AHL): 1 hour 40 multiple-choice questions on core and AHL, about 15 of which are common with SL.
The questions on paper 1 test assessment objectives 1, 2 and 3. The use of calculators is not permitted. Students will be provided with a periodic table. No marks are deducted for incorrect answers. Weighting: 20% (40 marks) Paper 2 (AHL): 2 hours 15 minutes Short-answer and extended-response questions on the core and AHL material. Calculators are allowed and a data booklet is provided. The questions on paper 2 test assessment objectives 1, 2 and 3. Free Online Textbooks, Math & Science Lesson Plans, Worksheets, Real World Examples & Teacher Resources.
Compound Interest. Copper(II) complexes and precipitates - Chemistry experiments - MEL Practicum. Solutions and precipitates of different color are obtained when blue solution is mixed with colorless solutions.
Scientific name: Copper ions combine easily with different ligands to form coordination complexes. Precipitates of different color are produced in reactions of copper sulfate with sodium salts. Reaction formula [Cu(H2O)6]2+ + 4NH3 → [Cu(H2O)2(NH3)4]2+ + 4H2O (blue) Mplex ions - colour. What about non-transition metal complex ions?

Non-transition metals don't have partly filled d orbitals. Visible light is only absorbed if some energy from the light is used to promote an electron over exactly the right energy gap. Non-transition metals don't have any electron transitions which can absorb wavelengths from visible light. For example, although scandium is a member of the d block, its ion (Sc3+) hasn't got any d electrons left to move around. This is no different from an ion based on Mg2+ or Al3+. In the zinc case, the 3d level is completely full - there aren't any gaps to promote an electron in to. Tetrahedral complexes Simple tetrahedral complexes have four ligands arranged around the central metal ion.
Gridlocks - Can you unlock the grid? CK-12 Foundation. Educational Innovations on Vimeo.
Schrödinger Wave Equation. Custom Search Dalton's Model of the Atom / J.J.
Thompson / Millikan's Oil Drop Experiment / Rutherford / Niels Bohr / DeBroglie / Heisenberg / Planck / Schrödinger / Chadwick Austrian physicist Erwin Schrödinger lays the foundations of quantum wave mechanics. In a series papers he describes his partial differential equation that is the basic equation of quantum mechanics and bears the same relation to the mechanics of the atom as Newton's equations of motion bear to planetary astronomy. Periodic table. Violations of the Octet Rule. Introduction to Molecular Orbital Theory. VSEPR. IB Chemistry on VSEPR.