

Uncertainty in Measurement. Online Text Even the most carefully taken measurements are always inexact.

This can be a consequence of inaccurately calibrated instruments, human error, or any number of other factors. Two terms are used to describe the quality of measurements: precision and accuracy. Precision is a measure of how closely individual measurements agree with one another. Accuracy refers to how closely individually measured numbers agree with the correct or "true" value.
Whatever the source, all measurements contain error. Consider a fourth-grade student who, when asked by his teacher how old the Earth is, replies "Four billion and three years old. " Volumetric. Lesson 2: Precision and Accuracy. In everyday language "precise" and "accurate” mean roughly the same thing... but not in physics.
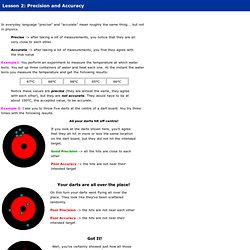
Precise -> after taking a lot of measurements, you notice that they are all very close to each other. Accurate -> after taking a lot of measurements, you find they agree with the true value Example1: You perform an experiment to measure the temperature at which water boils. You set up three containers of water and heat each one. At the instant the water boils you measure the temperature and get the following results: Notice these values are precise (they are almost the same, they agree with each other), but they are not accurate. Example 2: I ask you to throw five darts at the centre of a dart board. All your darts hit off centre! If you look at the darts shown here, you’ll agree that they all hit in more or less the same location on the dart board, but they did not hit the intended target. Your darts are all over the place! On this turn your darts went flying all over the place. Nope! Measurement Practice: Determining Proper Precision. Significant Figures.
Lesson Objectives The student will: explain the necessity for significant figures.
Determine significant figures of the equipment pieces chosen. Identify the number of significant figures in a measurement. Use significant figures properly in measurements and calculations. Vocabulary significant figures Introduction. Measurements. MEASUREMENTS: Water and aqueous solutions will form a concave meniscus when placed in a graduated cylinder as the water molecules are more strongly attracted to the glass than each other.

Reading a Ruler Measurement. Graduated cylinder module. OUR program The UW-Platteville Department of Chemistry is currently home to about 140 undergraduate majors, each of whom is pursuing a bachelor's degree in either chemistry, biochemistry, or criminalistics.
We have about 50 additional students in affiliated minors: chemistry and MEMS/nanotechnology. Our academic program is served by 10 faculty and several instructional staff, and we are supported by our administrative assistant, Kristina Sarris, our laboratory manager, Kari Frederick, and our stockroom assistant, Abbie Chadwick. UW-Platteville chemistry faculty represent the following areas of specialization: Analytical Chemistry and Criminalistics: Charles Cornett, Steven Steiner, and Joseph Wu Biochemistry: Jeff Buboltz and Chanaka Mendis Inorganic and Organometallic Chemistry: Timothy Zauche Organic Chemistry: June Li and Raja Annamalai Physical Chemistry: James Hamilton and Soma Chattopadhyay Our Facilities.
Reading the Volume from a 10-mL Graduated Cylinder. The Recording of Measurements. The Recording of Measurements A.

Accuracy and Precision Chemistry is an exact science; its development has been based on careful measurements of properties of matter and careful observations of changes in these properties. Measurements in chemistry must be both accurate and precise (Figure 2.4). An accurate measurement is one that is close to the actual value of the property being measured. The accuracy of a measurement depends on the calibration of the tool used to make the measurement. A precise measurement is one that can be reproduced. The importance of obtaining measurements that are both accurate and precise is rarely greater than in a medical laboratory. B. Mass of a hydrogen atom = 0.000 000 000 000 000 000 000 001 67 g At the opposite extreme is the mass of the Earth: Mass of the Earth = 5,976,000,000,000,000,000,000,000,000 g Such numbers are hard to deal with, difficult to copy without making mistakes, and almost impossible to name.
Recording Measurements. If you are going to understand significant digits, you have to be sure you are recording your measurements properly.

Sounds simple, right? And it is, yet it also has a few "gotcha's" to be careful about. For example, suppose that you were told to measure the length of the green line, using a normal centimetre ruler. Most of us wouldn't have much trouble at all indicating that the line is longer than 9 cm, but not quite 10. Therefore we would be absolutely positive in reporting that the line is between 9 and 10 cm long. Measurement Page. The officially uses the English system of measurements.

This is also referred to as the U. S. Customary system as well as the British Engineering System. The unit of length is FOOT whereas in the metric system, the unit of length is METER. The is the only industrialized country in the world that has NOT officially adopted the metric units as a standard. A measurement is defined as the ratio of the magnitude (how much) of any quantity to a standard value. In order to begin the study of a subject, first, we must establish a common language to be used by instructors and students when discussing that subject. Reading the Volume from a 100-mL Graduated Cylinder.