

Herbalism. Herbalism ("Herbology" or "Herbal Medicine") is use of plants for medicinal purposes, and the study of such use.
Plants have been the basis for medical treatments through much of human history, and such traditional medicine is still widely practiced today. Modern medicine recognizes herbalism as a form of alternative medicine, as the practice of herbalism is not strictly based on evidence gathered using the scientific method. List of plants used in herbalism. This is a list of plants that have been used as herbal medicine.
Plants have the ability to synthesize a wide variety of chemical compounds that are used to perform important biological functions, and to defend against attack from predators such as insects, fungi and herbivorous mammals. Many of these phytochemicals have beneficial effects on long-term health when consumed by humans, and can be used to effectively treat human diseases. Aromatherapy. Some essential oils such as tea tree[1] have demonstrated anti-microbial effects, but there is still a lack of clinical evidence demonstrating efficacy against bacterial, fungal, or viral infections.
Evidence for the efficacy of aromatherapy in treating medical conditions remains poor, with a particular lack of studies employing rigorous methodology,[2] but some evidence exists that essential oils may have therapeutic potential.[3] History[edit] List of essential oils. Davana Essential Oil.
Essential oil. An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants.
Tincture. Herbal tinctures are not always made using ethanol as the solvent, though this is most commonly the case. Other solvents include vinegar, glycerol, ether and propylene glycol, not all of which can be used for internal consumption. Ethanol has the advantage of being an excellent solvent for both acidic and basic (alkaline) constituents. Terpene. Many terpenes are derived commercially from conifer resins, such as those made by this pine.
Terpenes (/ˈtɜrpiːn/ TUR-peen) are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers,[1] though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeteria. They are often strong-smelling, and thus may protect the plants that produce them by deterring parasites. Many terpenes are aromatic hydrocarbons and thus may have had a protective function.
The difference between terpenes and terpenoids is that terpenes are hydrocarbons, whereas terpenoids contain additional functional groups. Glycoside. In chemistry, a glycoside /ˈɡlaɪkəsaɪd/ is a molecule in which a sugar is bound to another functional group via a glycosidic bond.
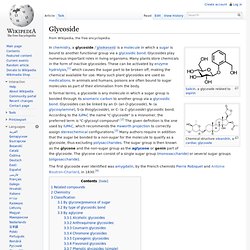
Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis,[1] which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. The first glycoside ever identified was amygdalin, by the French chemists Pierre Robiquet and Antoine Boutron-Charlard, in 1830.[4] Phyto- chemical.
Flowers Phytochemicals are chemical compounds that occur naturally in plants (phyto means "plant" in Greek).
Some are responsible for color and other organoleptic properties, such as the deep purple of blueberries and the smell of garlic. The term is generally used to refer to those chemicals that may have biological significance, for example antioxidants, but are not established as essential nutrients.[1] Scientists estimate[citation needed] that there may be as many as 10,000 different phytochemicals having the potential to affect diseases such as cancer, stroke or metabolic syndrome.
Phytochemicals as candidate nutrients[edit] Alkaloid. The boundary between alkaloids and other nitrogen-containing natural compounds is not clear-cut.[14] Compounds like amino acid peptides, proteins, nucleotides, nucleic acid, amines, and antibiotics are usually not called alkaloids.[2] Natural compounds containing nitrogen in the exocyclic position (mescaline, serotonin, dopamine, etc.) are usually classified as amines rather than as alkaloids.[15] Some authors, however, consider alkaloids a special case of amines.[16][17][18] Naming[edit] The article that introduced the concept of "alkaloid".
The name "alkaloids" (German: Alkaloide) was introduced in 1819 by the German chemist Carl Friedrich Wilhelm Meißner, and is derived from late Latin root Latin: alkali (which, in turn, comes from the Arabic al-qalwī – "ashes of plants") and the suffix Greek: -οειδής – "like". [nb 1] However, the term came into wide use only after the publication of a review article by Oscar Jacobsen in the chemical dictionary of Albert Ladenburg in the 1880s.[19][20]
Polyphenol. Plant-derived polyphenol, tannic acid, formed by esterification of ten equivalents of the phenylpropanoid-derived gallic acid to a monosaccharide (glucose) core from primary metabolism.
Neutral phenol substructure "shape". An image of a computed electrostatic surface of neutral phenol, showing neutral regions in green, electronegative areas in orange-red, and the electropositive phenolic proton in blue. Phenol-phenolate equilibrium, and resonance structures giving rise to phenolaromatic reactivity. See also the images at the wiki pages for phenols.