

Histidine. Histidine (abbreviated as His or H)[2] is an α-amino acid with an imidazole functional group.
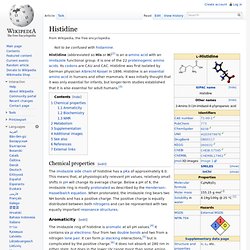
It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans and other mammals. It was initially thought that it was only essential for infants, but longer-term studies established that it is also essential for adult humans.[3] Chemical properties[edit] The imidazole side chain of histidine has a pKa of approximately 6.0. Aromaticity[edit] The imidazole ring of histidine is aromatic at all pH values.[4] It contains six pi electrons: four from two double bonds and two from a nitrogen lone pair.
Biochemistry[edit] The imidazole sidechain of histidine is a common coordinating ligand in metalloproteins and is a part of catalytic sites in certain enzymes. Certain amino acids can be converted to intermediates of the TCA cycle. Isoleucine. Isoleucine (abbreviated as Ile or I)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3.
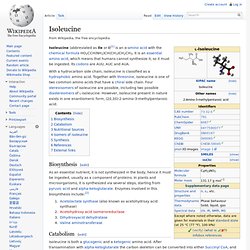
It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA. Biosynthesis[edit] As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acid and alpha-ketoglutarate. Leucine. Leucine (abbreviated as Leu or L)[2] is a branched-chain α-amino acid with the chemical formula HO2CCH(NH2)CH2CH(CH3)2.
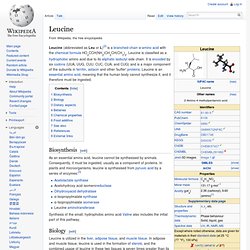
Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons (UUA, UUG, CUU, CUC, CUA, and CUG) and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins. Lysine. Lysine (abbreviated as Lys or K)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2.
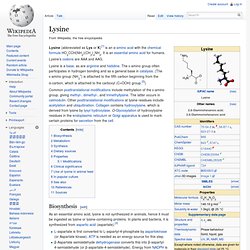
It is an essential amino acid for humans. Lysine's codons are AAA and AAG. Methionine. Methionine (/mɛˈθaɪ.ɵniːn/ or /mɛˈθaɪ.ɵnɪn/; abbreviated as Met or M)[3] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2SCH3.
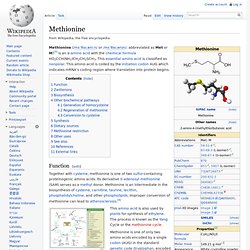
This essential amino acid is classified as nonpolar. This amino-acid is coded by the initiation codon AUG which indicates mRNA's coding region where translation into protein begins. Phenylalanine. Phenylalanine /ˌfɛn(ə)lˈæləˌniːn/ (abbreviated as Phe or F)[2] is an α-amino acid with the formula C6H5CH2CH(NH2)COOH.
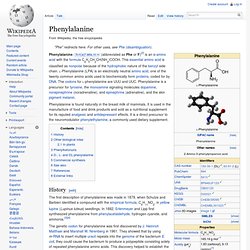
This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine (LPA) is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA. The codons for L-phenylalanine are UUU and UUC. Phenylalanine is a precursor for tyrosine, the monoamine signaling molecules dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin.
Phenylalanine is found naturally in the breast milk of mammals. History[edit] The first description of phenylalanine was made in 1879, when Schulze and Barbieri identified a compound with the empirical formula, C9H11NO2, in yellow lupine (Lupinus luteus) seedlings. The genetic codon for phenylalanine was first discovered by J. Threonine. Threonine (abbreviated as Thr or T)[2] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(OH)CH3.
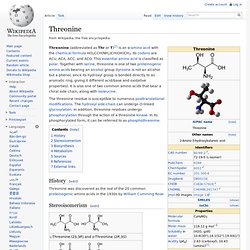
Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar. Together with serine, threonine is one of two proteinogenic amino acids bearing an alcohol group (tyrosine is not an alcohol but a phenol, since its hydroxyl group is bonded directly to an aromatic ring, giving it different acid/base and oxidative properties). It is also one of two common amino acids that bear a chiral side chain, along with isoleucine. The threonine residue is susceptible to numerous posttranslational modifications. History[edit] Threonine was discovered as the last of the 20 common proteinogenic amino acids in the 1930s by William Cumming Rose. Tryptophan. Tryptophan (IUPAC-IUBMB abbreviation: Trp or W; IUPAC abbreviation: L-Trp or D-Trp; sold for medical use as Tryptan)[2] is one of the 22 standard amino acids and an essential amino acid in the human diet, as demonstrated by its growth effects on rats.
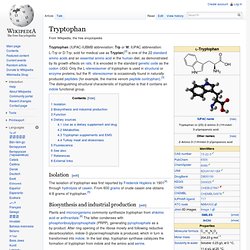
It is encoded in the standard genetic code as the codon UGG. Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the R -stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan).[3] The distinguishing structural characteristic of tryptophan is that it contains an indole functional group.
Isolation[edit] The isolation of tryptophan was first reported by Frederick Hopkins in 1901[4] through hydrolysis of casein. From 600 grams of crude casein one obtains 4-8 grams of tryptophan.[5] Biosynthesis and industrial production[edit] Function[edit] Metabolism of L-tryptophan into serotonin and melatonin (left) and niacin (right). Valine. Valine (abbreviated as Val or V)[3] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(CH3)2.
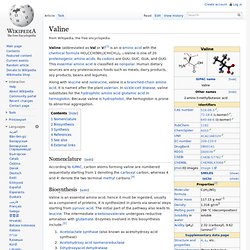
L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar. Human dietary sources are any proteinaceous foods such as meats, dairy products, soy products, beans and legumes. Nomenclature[edit] According to IUPAC, carbon atoms forming valine are numbered sequentially starting from 1 denoting the carboxyl carbon, whereas 4 and 4' denote the two terminal methyl carbons.[4] Essential amino acid. An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo (from scratch) by the organism being considered, and therefore must be supplied in its diet.
The amino acids regarded as essential for humans are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine.[1] Additionally, cysteine (or sulphur-containing amino acids), tyrosine (or aromatic amino acids), and arginine are always required by infants and growing children.[2][3] Essential amino acids are "essential" not because they are more important for protein synthesis or general homeostasis than the others, but because the body does not synthesize them. If they are not uptaken through diet, they will not be available for protein synthesis.