

James Muller
IONTOX is a biotechnology company founded in 2014 dedicated to providing expertise in the area of in vitro toxicology, with a mission to build improved methods for predicting human adverse effects from chemical exposure. From laboratories located in Kalamazoo, Michigan, IONTOX serves a global client base in the pharmaceutical, cosmetic, chemical, tobacco, and food additive industries.
A Basic Overview Of In Vitro IND Enabling Studies. Thousands of new compounds enter drug discovery and development each year.

Depending on the specific pharmaceutical compound, some are suitable for the market and some are not. Before any new drug becomes accessible to the public, in the United States, the new drug must be approved by the U.S. Food and Drug Administration (FDA). Part of being given U.S. FDA approval includes gaining acceptance to undergo human clinical trials to ensure the pharmaceutical compound will be safe for public use. The US FDA recommended IND Enabling studies focus on determining if a new drug candidate will be safe to proceed to human clinical trials. How Companies Begin IND Enabling Studies For An FDA IND Application.
What is Cytotoxicity Testing And Why Is It Necessary? IND Enabling Studies Research For The new Drug. Cytotoxicity Screening. Cytotoxicity Testing In The USA. In Vitro Toxicity Testing. Pharmaceutical, nutraceutical, food additive, and chemical companies need to know how their compounds affect the intestinal cell health.

Chemicals entering the intestinal tract can affect the intestinal epithelium. Compounds that enter the body by the oral route, enter the gastrointestinal tract (GIT). The GIT is a complex system that facilitates the digestion and absorption of food derived fats, carbohydrates, and proteins. The primary site of enzymatic breakdown and absorption is the upper portion of the small intestine (duodenum). Chemicals that enter the intestine via ingestion of medicines or food products are absorbed into the portal circulation and carried to the liver. The effects of chemicals on human intestinal heath must be evaluated using an in vitro model that is physiologically relevant.
Based on the questions being asked, IONTOX scientists will assist you in selecting the best in vitro model for your study. COVID-19 Antibody Test Kit. November 9, 2020 Ever since the Covid-19 hit the world, people haven’t talked about anything else except its causes, consequences and arrival of the vaccine.

A major impact was made to every sector of the economy. As the economy stumbled, the health sector became a critical area of analysis. The testing procedures that were followed were developed in order to increase its rate. Several come up with Covid-19 Antibody Test Kit, and the fact that every kit was laid under laboratory testing showed how important it was for this kit to display accuracy in its results. Prior to the solution, one must analyze what the real problem is! What Help Did It Provide To Tackle This Situation? • Clear Answer: The kits were made to generate results in a shorter period of time. COVID Antibody Test Kit. Coronavirus is spreading continually across the world.

This pandemic has been a challenge for everyone. The COVID-19 outbreak has changed our daily lives in ways we never could have imagined. Many companies use various screening methods to screen suspected COVID-19 individuals. As an increase in the number of cases, the technique of screening is not helping. Most countries are relying on a rapid COVID antibody test kit for testing COVID-19, which can give you results in just 15 minutes. Best Ways to Know the Symptoms of COVID-19. COVID-19 coronavirus outbreak has everyone spooked, and hopefully, taking steps to control the outbreak.
Most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment. But it is very dangerous or you can say deadly disease for those who are older and underlying several medical problems. This includes: Cardiovascular disease (Heart & stroke problems) Diabetes Chronic respiratory disease Cancer is more likely to develop serious illness.
Due to its speed and scale of transmission, COVID-19 is a pandemic. COVID-19 Antibody Test Kit. In this current scenario, all are aware of the coronavirus disease (Covid-19).
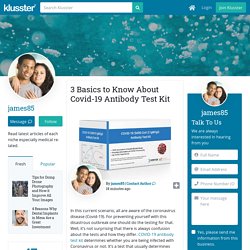
For preventing yourself with this disastrous outbreak one should do the testing for that. Well, it’s not surprising that there is always confusion about the tests and how they differ. COVID-19 antibody test kit determines whether you are being infected with Coronavirus or not. It’s a test that usually determines whether the person is currently suffering from this disease. Before proceeding further, let’s know what Antibody testing is? Coronavirus Antibody Test Kit – How To Test For Coronavirus? No country knows the total number of people infected with COVID-19.
All we know is the infection status of those who have been tested. All those who have a lab-confirmed infection are counted as confirmed cases. Well, A lot has been discussed about the recent outbreak of Coronavirus. But nobody knows exactly “What is COVID-19”? Coronavirus is a larger family of viruses that cause illnesses such as the common cold, severe acute respiratory syndrome (SARS), and the Middle East respiratory syndrome (MERS). Today, there are several kinds of Coronavirus antibody test kit launched by several industries. In case you feel your symptoms are specific to the coronavirus, your healthcare provider can get in touch with CDC or the local healthcare departments for testing instructions. 1: Swab Test: In this test, professionals use a special swab to take a sample from your nose or throat. 4: Sputum Test: In this case, Sputum is a thick mucus that gets accumulated in the lungs and comes out with a cough.