

Hallucinogen. Empathogen-entactogen. The terms empathogen and entactogen are used to describe a class of psychoactive drugs that produce distinctive emotional and social effects similar to those of MDMA (ecstasy).
Putative members of this class include 2C-B, 2C-I(at 2-14mg), MDMA, MDA, MDEA, MBDB and 6-APB among others. The chemical structure of many entactogens contains a substituted amphetamine core, and most belong to the phenethylamine class of psychoactive drugs, although several (AET and AMT) are tryptamines. When referring to MDMA and its counterparts, the term 'MDxx' is often used with the exception of MDPV.
Entactogens are sometimes incorrectly referred to as major hallucinogens or stimulants, which is untrue although their effects are often somewhat similar. [citation needed] Etymology[edit] The term "empathogen" was coined in 1983 by Ralph Metzner to denote chemical agents inducing feelings of empathy. Psychological effects[edit] Examples[edit] The chemicals below have a varying degree of entactogenic effects. Entheogen. A group of peyotes, in cultivation.
Peyote has been used in ritual contexts for thousands of years.[1][2][3] With the advent of organic chemistry, there now exist many synthetic drugs with similar psychoactive properties, many derived from these plants. Ibogaine. Ibogaine is a naturally occurring psychoactive substance found in plants in the Apocynaceae family such as Tabernanthe iboga, Voacanga africana and Tabernaemontana undulata.
A psychedelic with dissociative properties, the substance is banned in some countries; in other countries it is used by proponents of psychedelic therapy to treat addiction to methadone, heroin, alcohol, cocaine, methamphetamine, anabolic steroids, and other drugs. Ibogaine is also used to treat depression and post traumatic stress disorder. Derivatives of ibogaine that lack the substance's psychedelic properties are under development.[1] Ibogaine-containing preparations are used for medicinal and ritual purposes within African spiritual traditions of the Bwiti, who claim to have learned it from the Pygmy peoples.
Although it was first commonly advertised as having anti-addictive properties in 1962 by Howard Lotsof, its western use predates that by at least a century. Psychedelic drug. LSD is widely known as a psychedelic drug and often features psychedelic artwork on its blotters A psychedelic substance is a psychoactive drug whose primary action is to alter cognition and perception.
Psychedelics are part of a wider class of psychoactive drugs known as hallucinogens, a class that also includes structurally unrelated substances such as dissociatives and deliriants. Unlike other drugs such as stimulants and opioids which induce familiar states of consciousness, psychedelics tend to affect and explore the mind in ways that result in the experience being qualitatively different from those of ordinary consciousness. Ayahuasca. Ayahuasca (UK: /ˌaɪ(j)əˈwæskə/, US: /-ˈwɑːskə/) or ayaguasca[1] (in Hispanicized spellings) from Quechua Ayawaska[2] (aya: soul, waska: vine), or yagé (/jɑːˈheɪ, jæ-/), is an entheogenic brew made out of Banisteriopsis caapi vine and other ingredients.[3] The brew is used as a traditional spiritual medicine in ceremonies among the indigenous peoples of the Amazon basin and is known by a number of different names (see below).[4] B. caapi contains several alkaloids that act as monoamine oxidase inhibitors (MAOIs).
Another common ingredient in ayahuasca is the shrub Psychotria viridis which contains the primary psychoactive, dimethyltryptamine (DMT). MAOIs are required for DMT to be orally active.[5] Nomenclature[edit] Ayahuasca is known by many names throughout Northern South America and Brazil. Lysergic acid diethylamide. Lysergic acid diethylamide, abbreviated LSD or LSD-25, also known as lysergide (INN) and colloquially as acid, is a semisynthetic psychedelic drug of the ergoline family, well known for its psychological effects which can include altered thinking processes, closed- and open-eye visuals, synesthesia, an altered sense of time and spiritual experiences, as well as for its key role in 1960s counterculture.
It is used mainly as an entheogen, recreational drug, and as an agent in psychedelic therapy. LSD is non-addictive, is not known to cause brain damage, and has extremely low toxicity relative to dose.[3] However, acute adverse psychiatric reactions such as anxiety, paranoia, and delusions are possible.[4] LSD was first synthesized by Albert Hofmann in 1938 from ergotamine, a chemical derived by Arthur Stoll from ergot, a grain fungus that typically grows on rye. Psilocybin Mushroom Vault: Basics. There are dozens of species of psilocybin or 'magic mushrooms' belonging primarily to the genuses psilocybe, panaeolus, and copelandia (unrelated to psychoactive amanita species).
The effects of their ingestion resemble a shorter acting LSD trip, producing significant physical, visual, and perceptual changes. Nearly all of the psilocybin containing mushrooms are small brown or tan mushrooms easily mistakable for any number of non-psychoactive, inedible, or poisonous mushrooms in the wild. This makes them somewhat difficult, and potentially hazardous, to identify. The primary distinguishable feature of most psilocybin containing mushrooms is that they bruise blue when handled. Recreational doses range from 1-5 grams of dry mushrooms depending on the species and individual strength of the specimens. . $20-$40 per 1/8 ounce. $100 - $250 per ounce. The primary effects of mushrooms come from several active alkaloids they contain; the most common are psilocybin, psilocin, and baeocystin.
Psilocybin. Psilocybin[nb 1] (/ˌsɪləˈsaɪbɪn/ SIL-ə-SY-bin) is a naturally occurring psychedelic compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms.
The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. 25I-NBOMe. 25I-NBOMe (2C-I-NBOMe, Cimbi-5) is a psychedelic drug and derivative of the substituted phenethylamine psychedelic 2C-I. It was discovered in 2003 by chemist Ralf Heim at the Free University of Berlin, who published his findings in his PhD dissertation.[1] The compound was subsequently investigated by a team at Purdue University led by David Nichols.[2] 25I-NBOMe. 25I-NBOMe is derived from the psychedelic phenethylamine 2C-I by substitution on the amine with a 2-methoxybenzyl (BOMe) group.
[hide] - [top]Ways of Administration Common methods of administration include sublingual and intranasal. The doses are typically volumetrically dosed due to the extremely low dosage for activity (microgram ranges). The dosing method commonly used is liquid or blotter form. The HCl form is commonly found from RC vendors along with pre-dosed blotters and liquid form already volumetrically dosed using ethanol. 2C-I-NBOMe (25I-NBOMe) Vault. Dimethyltryptamine. History[edit] Another historical milestone is the discovery of DMT in plants frequently used by Amazonian natives as additive to the vine Banisteriopsis caapi to make ayahuasca decoctions.
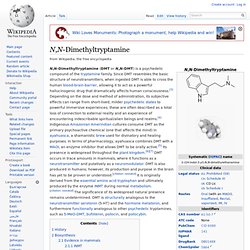
Biosynthesis[edit] Biosynthetic pathway for N,N-dimethyltryptamine This transmethylation mechanism has been repeatedly and consistently proven by radiolabeling of SAM methyl group with carbon-14 (14C-CH3)SAM).[22][20][24][25][26] DMT (Dimethyltryptamine) Vault. Dissociative. Classes of dissociatives[edit] NMDA receptor antagonists[edit] κ-opioid receptor agonists[edit] Effects[edit] The effects of dissociatives can include sensory dissociation, hallucinations, mania, catalepsy, analgesia and amnesia.[9][10][11] The characteristic features of dissociative anesthesia were described as catalepsy, amnesia and analgesia.[9] According to Pender (1972), "the state has been designated as dissociative anesthesia since the patient truly seems disassociated from his environment.
Deliriant. Deliriants are a class of hallucinogen. The term was introduced by David F. Duncan and Robert S. Dextromethorphan. Dextromethorphan (DXM or DM) is an antitussive (cough suppressant) drug. It is one of the active ingredients in many over-the-counter cold and cough medicines, including generic labels and store brands, Benylin DM, Mucinex DM, Robitussin, NyQuil, Dimetapp, Vicks, Coricidin, Delsym, TheraFlu, and others. Dextromethorphan has also found other uses in medicine, ranging from pain relief to psychological applications. It is sold in syrup, tablet, spray, and lozenge forms.
In its pure form, dextromethorphan occurs as a white powder.[2] DXM is also used recreationally. Medical use[edit] The primary use of dextromethorphan is as a cough suppressant, for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the flu and common cold), as well as those resulting from inhaled particle irritants. DXM (Dextromethorphan, DM) Vault. Tetrahydrocannabinol. Tetrahydrocannabinol (THC), or more precisely its main isomer (−)-trans-Δ9-tetrahydrocannabinol ( (6aR,10aR)-delta-9-tetrahydrocannabinol), is the principal psychoactive constituent (or cannabinoid) of the cannabis plant.
First isolated in 1964, in its pure form, by Israeli scientists Raphael Mechoulam and Yechiel Gaoni at the Weizmann Institute of Science,[8][9][10] it is a glassy solid when cold, and becomes viscous and sticky if warmed. A pharmaceutical formulation of (−)-trans-Δ9-tetrahydrocannabinol, known by its INN dronabinol, is available by prescription in the U.S. and Canada under the brand name Marinol.
An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents, specifically lipids and alcohols.[6] Tetrahydrocannabinol with double bond isomers and their stereoisomers is one of only three cannabinoids scheduled by Convention on Psychotropic Substances (the other two are dimethylheptylpyran and parahexyl). Endocannabinoid system. The endocannabinoid system has been studied using genetic and pharmacological methods.
These studies have revealed that cannabinoids act as neuromodulators[2][3][4] for a variety of physiological processes, including motor learning,[5] synaptic plasticity,[6] appetite,[7] and pain sensation.[8] Serotonin. Serotonin /ˌsɛrəˈtoʊnɨn/ or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. NMDA receptor antagonist. Ketamine, one of the most common NMDA receptor antagonists.