

Glycerol. Glycerol (or glycerine, glycerin) is a simple polyol (sugar alcohol) compound.
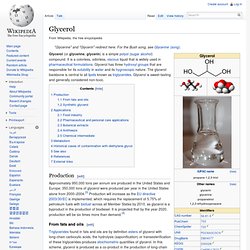
It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids known as triglycerides. Glycerol is sweet-tasting and generally considered non-toxic. Production[edit] Approximately 950,000 tons per annum are produced in the United States and Europe; 350,000 tons of glycerol were produced per year in the United States alone from 2000–2004.[3] Production will increase as the EU directive 2003/30/EC is implemented, which requires the replacement of 5.75% of petroleum fuels with biofuel across all Member States by 2010, as glycerol is a byproduct in the production of biodiesel.
From fats and oils[edit] It is also a byproduct of the production of biodiesel via transesterification. Synthetic glycerol[edit] Applications[edit] [edit] Sodium peroxide. Disodium pyrophosphate. Disodium pyrophosphate or sodium acid pyrophosphate is an inorganic compound consisting of sodium cations and pyrophosphate anion.
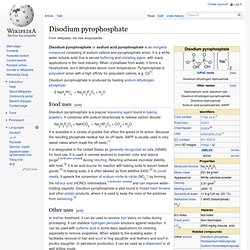
It is a white, water-soluble solid that is serves buffering and chelating agent, with many applications in the food industry. When crystallised from water, it forms a hexahydrate, but it dehydrates above room temperature. Pyrophosphate is polyvalent anion with a high affinity for polyvalent cations, e.g. Ca2+. Disodium pyrophosphate is produced by heating sodium dihydrogen phosphate: 2 NaH2PO4 → Na2H2P2O7 + H2O Food uses[edit] Disodium pyrophosphate is a popular leavening agent found in baking powders. Na2H2P2O7 + NaHCO3 → Na3HP2O7 + CO2 + H2O It is available in a variety of grades that affect the speed of its action.
It is designated in the United States as generally recognized as safe (GRAS) for food use. Other uses[edit] In leather treatment, it can be used to remove iron stains on hides during processing. References[edit] Sodium pyrophosphate decahydrate. Sodium Pyrophosphate, Decahydrate, Crystal, Reagent, ACS * 13472-36-1. Polyethylene Glycols (PEGs) Sheftel, VO.
Indirect Food Additives and Polymers: Migration and Toxicology. Lewis 2000 pp.1114-1116 Structural Formula. HO[~CH2CH2O~]nH M = 500,000 to 10,000000 CAS No 25322-68-3 RTECS No TQ3500000 Abbreviation. PEGs. Synonyms and Trade Names. Properties. Applications. Migration of up to 50 mg PEGs/kg food was observed in chocolates, boiled sweets, toffees, cakes, and meat pies that were wrapped in regenerated cellulose films containing various mixtures of glycol softeners. Acute Toxicity. The mean lethal doses of PEG are presented in the table.03 Table Mean lethal doses of PEGs (g/kg BW).