

Thermodynamics. Annotated color version of the original 1824 Carnot heat engine showing the hot body (boiler), working body (system, steam), and cold body (water), the letters labeled according to the stopping points in Carnot cycle Thermodynamics applies to a wide variety of topics in science and engineering.
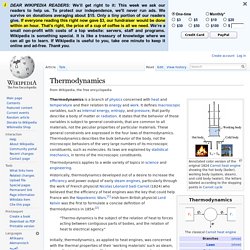
Historically, thermodynamics developed out of a desire to increase the efficiency and power output of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot (1824) who believed that the efficiency of heat engines was the key that could help France win the Napoleonic Wars.[1] Irish-born British physicist Lord Kelvin was the first to formulate a concise definition of thermodynamics in 1854:[2] "Thermo-dynamics is the subject of the relation of heat to forces acting between contiguous parts of bodies, and the relation of heat to electrical agency. " Introduction[edit] A thermodynamic system can be defined in terms of its states. Quantum mechanics. Solution to Schrödinger's equation for the hydrogen atom at different energy levels.
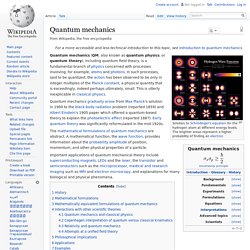
The brighter areas represent a higher probability of finding an electron Quantum mechanics gradually arose from Max Planck's solution in 1900 to the black-body radiation problem (reported 1859) and Albert Einstein's 1905 paper which offered a quantum-based theory to explain the photoelectric effect (reported 1887). Particle physics. Subatomic particles[edit] Modern particle physics research is focused on subatomic particles, including atomic constituents such as electrons, protons, and neutrons (protons and neutrons are composite particles called baryons, made of quarks), produced by radioactive and scattering processes, such as photons, neutrinos, and muons, as well as a wide range of exotic particles.
Dynamics of particles is also governed by quantum mechanics; they exhibit wave–particle duality, displaying particle-like behavior under certain experimental conditions and wave-like behavior in others. In more technical terms, they are described by quantum state vectors in a Hilbert space, which is also treated in quantum field theory. Following the convention of particle physicists, the term elementary particles is applied to those particles that are, according to current understanding, presumed to be indivisible and not composed of other particles.[1]
Concepts. Subtopics. Nuclear physics. Condensed matter physics. The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists,[2] and The Division of Condensed Matter Physics (DCMP) is the largest division of the American Physical Society.[3] The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics.
Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.[4] References to "condensed" state can be traced to earlier sources. For example, in the introduction to his 1947 "Kinetic theory of liquids" book,[8] Yakov Frenkel proposed that "The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies. History[edit]
Major theories. Sub fields. Classical physics. The four major domains of modern physics Classical physics refers to theories of physics that predate modern, more complete, or more widely applicable theories.
If a currently accepted theory is considered to be "modern," and its introduction represented a major paradigm shift, then previous theories (or new theories based on the older paradigm) will often be referred to as "classical". As such, the definition of a classical theory depends on context. Classical physical concepts are often used when modern theories are unnecessarily complex for a particular situation. Chemical physics. Chemical physics is a subdiscipline of chemistry and physics that investigates physicochemical phenomena using techniques from atomic and molecular physics and condensed matter physics; it is the branch of physics that studies chemical processes from the point of view of physics.
While at the interface of physics and chemistry, chemical physics is distinct from physical chemistry in that it focuses more on the characteristic elements and theories of physics. Meanwhile, physical chemistry studies the physical nature of chemistry. Nonetheless, the distinction between the two fields is vague, and workers often practice in each field during the course of their research.[1] What chemical physicists do[edit] Journals[edit] See also[edit]
Biophysics. Photosynthetic reaction center.
Biophysics is an interdisciplinary science using methods of, and theories from, physics to study biological systems.[1] Biophysics spans all levels of biological organization, from the molecular scale to whole organisms and ecosystems. Biophysical research shares significant overlap with biochemistry, nanotechnology, bioengineering, agrophysics, and systems biology. It has been suggested as a bridge between biology and physics.
Overview[edit] Molecular biophysics typically addresses biological questions similar to those in biochemistry and molecular biology, but more quantitatively. Fluorescent imaging techniques, as well as electron microscopy, x-ray crystallography, NMR spectroscopy and atomic force microscopy (AFM) are often used to visualize structures of biological significance. Astrophysics. Astrophysics (from Greek astron, ἄστρον "star", and physis, φύσις "nature") is the branch of astronomy that deals with the physics of the universe, especially with "the nature of the heavenly bodies, rather than their positions or motions in space.
Major Theories. Sub fields.