

Homemade Shampoo Soap Bars Recipe and Instructions. By David Fisher Updated December 20, 2014.
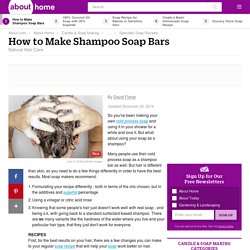
So you've been making your own cold process soap and using it in your shower for a while and love it. But what about using your soap as a shampoo? Many people use their cold process soap as a shampoo bar as well. But hair is different than skin, so you need to do a few things differently in order to have the best results. Formulating your recipe differently - both in terms of the oils chosen, but in the additives and superfat percentageUsing a vinegar or citric acid rinseKnowing that some people's hair just doesn't work well with real soap - and being o.k. with going back to a standard surfactant-based shampoo. RECIPESFirst, for the best results on your hair, there are a few changes you can make to your regular soap recipe that will help your soap work better on hair. Continue reading below our video Play Video Here are three recipes to get you started. I list the percentages as well as the ingredients to make a 2 lb batch of soap. Soap Making Tips & Tricks.
The following are several Tips & Tricks submitted by Oregon Trail Customers (and a few by me!).
We would love to hear your own personal Tips & Tricks and even add them to our resource list. If you have a great tip or trick to submit, please click on the "Submit Tip" button on this page or send us an email through our "Contact Us" page. Tips from Suz Q: How much flavor should I use when making Lip Products? Rosemary Mint Shampoo Bar Recipe {Video Tutorial} Troubleshooting Help! Botched Batches! Miller's Homemade Soap Pages: *APOLOGY* ... to those of you who have emailed me and have never heard a peep out of me...

I'm sorry. I don't have as much time for personal email responses as I once did and added to that have been occasional travel, garden catch-up, church responsibilities, soapmaking and orders and more visits from children and grandchildren who have moved nearby. I just can't keep up. If your email involved troubleshooting of a recipe or a lengthy response, it was probably put aside for when there was more time and then fell through the cracks because "more time" didn't happen. :-/ I don't see that changing in the near future. If you'd like to contribute your experiences and/or frustrations to this page, just send an email and I'll post it if I think it would be helpful to others or offers new information. Goat Milk. About - Making Soap Mag. Making Soap, Cosmetics & Candles — the Magazine Satisfying one’s curiosity begets opportunity; acting upon opportunity opens doors of enlightenment and motivation, which results in creating something truly novel and useful, ultimately uniting a community.
This is what happened. It all started back in 1996 when Kathy Tarbox (the publisher) encountered “essential oils” in an article she read. So intrigued, she took to the information superhighway, where, essentially, she had read a bit about essential oils in soap making, and was inspired by the first internet soap-list. By the end of that year, she started her soap business (Petal Pusher Botanical Soap Co.), and began selling her soap. She wanted more information, but there was nothing out there that guided independent soapmakers. The magazine serves both the professional as well as aspiring soap, cosmetic & candle makers.
View Past Issues! Soap and Saponification - Chemistry. Preparation & Chemical Structure One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Hydrolysis of the fats and oils occurs, yielding glycerol and crude soap. In the industrial manufacture of soap, tallow (fat from animals such as cattle and sheep) or vegetable fat is heated with sodium hydroxide. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. The crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide, and glycerol. Soap Making: Learn how to make soap. Cranberry Lane Make-it-Yourself Bodycare, Soap and Soapmaking Supplies, Soapmaking Kits, Essential Oils, Herbs, Waxes, Molds, Lye Calculator, and Kits.
Cranberry Lane's Lye Calculator Simply enter the weights of the oils or waxes in your soap formula and the calculator will determine the amount of Sodium Hydroxide and Water required.
Units Neither the author nor the company can be held responsible for damage, injury or otherwise resulting from the use of the lye calculator, formulas, information or materials listed. Basic Soap Making Recipes - Make Natural Homemade Soap. Saponification-The process of Making Soap (Theory) : Class 10 : Chemistry : Amrita Online Lab. To study the saponification reaction for preparation of soap.

Soaps and detergents are essential to personal and public health. They safely remove germs, soils and other contaminants and help us to stay healthy and make our surroundings more pleasant. Soaps are made from fats and oils or their fatty acids. What are fatty acids? Fatty acids are merely carboxylic acids consisting of a long hydrocarbon chain at one end and a carboxyl group (-COOH) at the other end.
Saturated fatty acids:Fatty acids contain carbon-carbon single bonds called saturated fatty acids. Unsaturated fatty acids:Unsaturated fatty acids contain one or more double bonds between carbon atoms. If the fatty acid has a single carbon-carbon double bond in the molecule, it is known as a mono-unsaturated fatty acid. If a fatty acid has two or more carbon-carbon double bonds in the molecule, it is known as poly-unsaturated fatty acid. Linoleic acid { CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH } is a poly-unsaturated fatty acid.