

Hotmail - martin_dery. Merck Index. Solvents. WebElements Periodic Table of the Elements. Bond Lengths and Energies. CAcT HomePage Skills to develop Define bondlength and bond energy and note relationship between the two Define bond order explain its relationship to bondlength or bond energy Evaluate enthalpies of reactions using bond energies Recognize covalent substances and characterize ionic character as difference in electonegativity Describe trends in bondlengths of a series of related compounds Distances between centers of bonded atoms are called bondlengths, or bond distances.
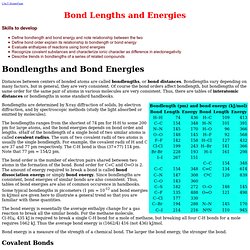
Bondlengths vary depending on many factors, but in general, they are very consistent. Of course the bond orders affect bondlength, but bondlengths of the same order for the same pair of atoms in various molecules are very consistent. Energy Conversion. Evans_pKa_table.pdf (application/pdf Object) Organic Division Information. KEGG PATHWAY Database.