

Emoxypine. Emoxypine was first synthesized by L.D.
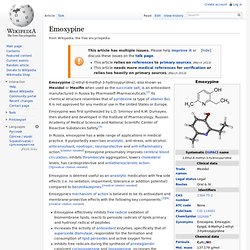
Smirnov and K.M. Meclofenoxate. BAY 73-6691. BAY 73-6691 is a drug developed by Bayer for the treatment of Alzheimer's disease.
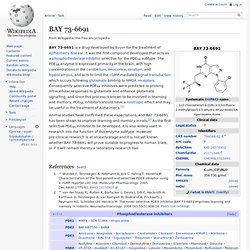
It was the first compound developed that acts as a phosphodiesterase inhibitor selective for the PDE9A subtype. The PDE9A enzyme is expressed primarily in the brain, with high concentrations in the cerebellum, neocortex, striatum, and hippocampus, and acts to limit the cGMP-mediated signal transduction which occurs following glutamate binding to NMDA receptors. Consequently selective PDE9A inhibitors were predicted to prolong intracellular responses to glutamate and enhance glutamate signalling, and since this process is known to be involved in learning and memory, PDE9A inhibitors should have a nootropic effect and may be useful in the treatment of Alzheimer's.[1] Jump up ^ Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M.
Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Ensaculin. Ensaculin (KA-672) is a drug from the coumarin family, which has been researched as a potential treatment for dementia.
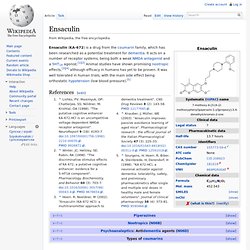
It acts on a number of receptor systems, being both a weak NMDA antagonist and a 5HT1A agonist.[1][2] Animal studies have shown promising nootropic effects,[3][4] although efficacy in humans has yet to be proven. It was well tolerated in human trials, with the main side effect being orthostatic hypotension (low blood pressure).[5] Jump up ^ Lishko, PV; Maximyuk, OP; Chatterjee, SS; Nöldner, M; Krishtal, OA (1998). "The putative cognitive enhancer KA-672.HCl is an uncompetitive voltage-dependent NMDA receptor antagonist".
NeuroReport 9 (18): 4193–7. doi:10.1097/00001756-199812210-00035. Galantamine. Galantamine (Nivalin, Razadyne, Razadyne ER, Reminyl, Lycoremine) is used for the treatment of mild to moderate Alzheimer's disease and various other memory impairments, in particular those of vascular origin.
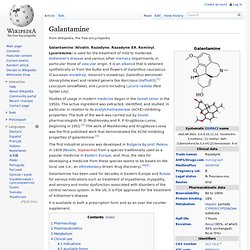
It is an alkaloid that is obtained synthetically or from the bulbs and flowers of Galanthus caucasicus (Caucasian snowdrop, Voronov's snowdrop), Galanthus woronowii (Amaryllidaceae) and related genera like Narcissus (daffodil)),[1] Leucojum (snowflake), and Lycoris including Lycoris radiata (Red Spider Lily). Studies of usage in modern medicine began in the Soviet Union in the 1950s. The active ingredient was extracted, identified, and studied, in particular in relation to its acetylcholinesterase (AChE)-inhibiting properties. The bulk of the work was carried out by Soviet pharmacologists M. D. The first industrial process was developed in Bulgaria by prof. It is available in both a prescription form and as an over the counter supplement.
Bifemelane. S-17092. S-17092 is a drug which acts as a selective inhibitor of the enzyme Prolyl endopeptidase.[1] This enzyme is involved in the metabolic breakdown of a number of neuropeptide neurotransmitters in the brain,[2][3] and so inhibiting the action of the enzyme increases the activity of these neuropeptides.
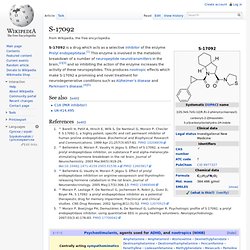
This produces nootropic effects which make S-17092 a promising and novel treatment for neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.[4][5] See also[edit] References[edit] Linopirdine. Linopirdine is a putative cognition-enhancing drug with a novel mechanism of action.
Calea ternifolia. It is used in traditional medicine and ritual in its native range.[3] Uses[edit] In Mexico the plant is used as an herbal remedy for dysentery and fever.[3] The Zoque Popoluca people call the plant tam huñi ("bitter gum") and use it to treat diarrhea and asthma, and the Mixe people know it as poop taam ujts ("white bitter herb") and use it for stomachache and fever.[4] The Chontal people of Oaxaca reportedly use the plant, known locally as thle-pela-kano, during divination.
Isolated reports describe rituals that involve smoking a plant believed to be this species, drinking it as a tea, and placing it under a pillow to induce divinatory dreams.
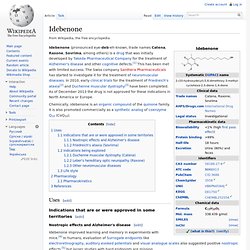
Fipexide. Idebenone. Idebenone (pronounced eye-deb-eh-known, trade names Catena, Raxone, Sovrima, among others) is a drug that was initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer's disease and other cognitive defects.[1] This has been met with limited success.
The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of neuromuscular diseases. In 2010, early clinical trials for the treatment of Friedreich's ataxia[2] and Duchenne muscular dystrophy[3] have been completed. As of December 2013[update] the drug is not approved for these indications in North America or Europe.
Chemically, idebenone is an organic compound of the quinone family.