

BBC Weather - Ozone layer hole: How its discovery changed our lives. 17 May 2015Last updated at 15:25 By Laurence Cawley BBC News The largest hole in the ozone layer appears over Antarctica Thirty years ago three scientists from Cambridge found a hole in the ozone layer, a shield high in the sky protecting us from potentially lethal solar radiation.

It changed the way we live in several important ways, ranging from international relations to beauty products. Jonathan Shanklin, together with Brian Gardiner and the late Joe Farman, was part of the British Antarctic Survey team which discovered the hole in the ozone layer in 1985. "I suppose I have changed the world," he says, citing one of the greatest legacies of his discoveries as "how fragile our environment is and that we tamper with it at our peril". The response to this peril was bold, decisive and occasionally surprising.
War paint and rickets The Australians were ahead of the pack when it came to sun protection. "Australia were significantly ahead of us," she says. Global Greenhouse Gas Emissions. Key Points In 2010, estimated worldwide emissions from human activities totaled nearly 46 billion metric tons of greenhouse gases, expressed as carbon dioxide equivalents.
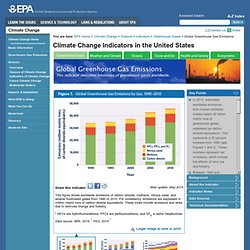
This represents a 35 percent increase from 1990 (see Figures 1 and 2). These numbers represent net emissions, which include the effects of land use and forestry. Between 1990 and 2010, global emissions of all major greenhouse gases increased (see Figure 1). Net emissions of carbon dioxide increased by 42 percent, which is particularly important because carbon dioxide accounts for about three-fourths of total global emissions. Background. Greenhouse Gases. Greenhouse gases from human activities are the most significant driver of observed climate change since the mid-20th century.1 The indicators in this chapter characterize emissions of the major greenhouse gases resulting from human activities, the concentrations of these gases in the atmosphere, and how emissions and concentrations have changed over time.

When comparing emissions of different gases, these indicators use a concept called “global warming potential” to convert amounts of other gases into carbon dioxide equivalents. Why does it matter? As greenhouse gas emissions from human activities increase, they build up in the atmosphere and warm the climate, leading to many other changes around the world—in the atmosphere, on land, and in the oceans. The indicators in other chapters of this report illustrate many of these changes.
These changes have both positive and negative effects on people, society, and the environment—including plants and animals. Summary of Key Points U.S. Causes of Climate Change. Key Points Both natural and human factors change Earth’s climate.

Before humans, changes in climate resulted entirely from natural causes such as changes in Earth’s orbit, changes in solar activity, or volcanic eruptions. Since the Industrial Era began, humans have had an increasing effect on climate, particularly by adding billions of tons of heat-trapping greenhouse gases to the atmosphere. Most of the observed warming since the mid-20th century is due to human-caused greenhouse gas emissions. Earth’s temperature is a balancing act Earth’s temperature depends on the balance between energy entering and leaving the planet’s system . View enlarged image. Ozone Layer on Track to Recovery. Nairobi/Geneva, 10 September 2014 (UNEP/WMO) – The Earth’s protective ozone layer is well on track to recovery in the next few decades thanks to concerted international action against ozone depleting substances, according to a new assessment by 300 scientists.

Ozone-reports-6-oct-2014.pdf. Class I Ozone-depleting Substances. Ozone and the ozone layer Australia. What is the ozone layer?
Ozone is a naturally occurring molecule containing three atoms of oxygen. Ozone molecules form a gaseous layer mostly in the upper atmosphere (the stratosphere) 15-30 km above the surface of the earth, and protects life on earth by absorbing ultra-violet (UV) radiation from the sun. The stratospheric ozone layer protects life on Earth by absorbing ultra-violet (UV) radiation from the sun. UV radiation is linked to skin cancer, genetic damage and immune system suppression in living organisms, and reduced productivity in agricultural crops and the food chain. Scientific evidence has proven that the natural balance of stratospheric ozone has been upset by the production and release into the atmosphere of ozone depleting substances, including chlorofluorocarbons CFC, halons, CH3CCl3 (Methyl chloroform), carbon tetrachloride, hydrochlorofluorocarbons (HCFCs) and methyl bromide.