

Titanium dioxide. Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium, chemical formula TiO 2.
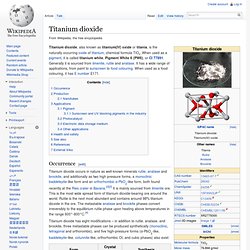
When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. Generally it is sourced from ilmenite, rutile and anatase. It has a wide range of applications, from paint to sunscreen to food colouring. When used as a food colouring, it has E number E171. Occurrence[edit] Titanium dioxide occurs in nature as well-known minerals rutile, anatase and brookite, and additionally as two high pressure forms, a monoclinic baddeleyite-like form and an orthorhombic α-PbO2-like form, both found recently at the Ries crater in Bavaria.[2][3] It is mainly sourced from ilmenite ore.
The cotunnite-type phase was claimed by L. Spectral lines from titanium oxide are prominent in class M stars, which are cool enough to allow molecules of this chemical to form. Production[edit] Rutile is the second most abundant mineral sand. Superhydrophilicity. Anatase. Anatase is one of the three mineral forms of titanium dioxide, the other two being brookite and rutile.
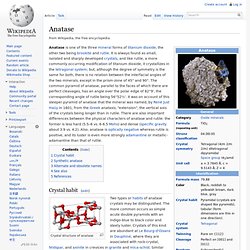
It is always found as small, isolated and sharply developed crystals, and like rutile, a more commonly occurring modification of titanium dioxide, it crystallizes in the tetragonal system; but, although the degree of symmetry is the same for both, there is no relation between the interfacial angles of the two minerals, except in the prism-zone of 45° and 90°. The common pyramid of anatase, parallel to the faces of which there are perfect cleavages, has an angle over the polar edge of 82°9', the corresponding angle of rutile being 56°52½'. Advanced oxidation process.
Advanced oxidation processes (abbreviation: AOPs), in a broad sense, refers to a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and waste water by oxidation through reactions with hydroxyl radicals (·OH).[1] In real-world applications of wastewater treatment, however, this term usually refers more specifically to a subset of such chemical processes that employ ozone (O3), hydrogen peroxide (H2O2) and/or UV light.[2] One such type of process is called in situ chemical oxidation.
Description[edit] AOPs rely on in-situ production of highly reactive hydroxyl radicals (·OH). These reactive species are the strongest oxidants that can be applied in water and can virtually oxidize any compound present in the water matrix, often at a diffusion controlled reaction speed. Consequently, ·OH reacts unselectively once formed and contaminants will be quickly and efficiently fragmented and converted into small inorganic molecules. Scheme 1. Photocatalysis. In the experiment above, photons from a light source (out of frame on the right hand side) are absorbed by the surface of the titanium dioxide disc, exciting electrons within the material.
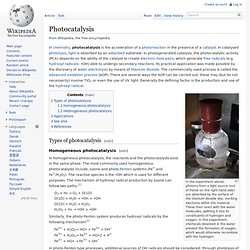
These then react with the water molecules, splitting it into its constituents of hydrogen and oxygen. In this experiment, chemicals dissolved in the water prevent the formation of oxygen, which would otherwise recombine with the hydrogen. Types of photocatalysis[edit] Homogeneous photocatalysis[edit] In homogeneous photocatalysis, the reactants and the photocatalysts exist in the same phase. O3 + hν → O2 + O(1D) H2O2 + hν → •OH + •OH Similarly, the photo-Fenton system produces hydroxyl radicals by the following mechanism[2] Fe2+ + H2O2→ HO• + Fe3+ + OH− Adams's catalyst. Adams's catalyst, also known as platinum dioxide, is usually represented as platinum(IV) oxide hydrate, PtO2•H2O.
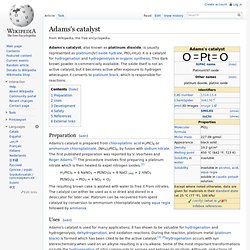
It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions. Hydrogenation. Because of the importance of hydrogen, many related reactions have been developed for its use.
Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative sources of hydrogen, not H2: these processes are called transfer hydrogenations. The reverse reaction, removal of hydrogen from a molecule, is called dehydrogenation. A reaction where bonds are broken while hydrogen is added is called hydrogenolysis, a reaction that may occur to carbon-carbon and carbon-heteroatom (oxygen, nitrogen or halogen) bonds.
Hydrogenation differs from protonation or hydride addition: in hydrogenation, the products have the same charge as the reactants. Hydrogenation of unsaturated fats produces saturated fats. Photocatalysis. Hydrolysis. Hydrolysis (/haɪˈdrɒlɨsɪs/; from Greek hydro-, meaning "water", and lysis, meaning "separation") usually means the cleavage of chemical bonds by the addition of water.
Where a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g. sucrose being broken down into glucose and fructose), this is termed saccharification. Generally, hydrolysis or saccharification is a step in the degradation of a substance. Redox. The two parts of a redox reaction Redox (portmanteau of reduction and oxidation) reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species.
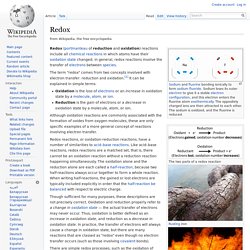
The term "redox" comes from two concepts involved with electron transfer: reduction and oxidation.[1] It can be explained in simple terms: