

Great Smog. The Great Smog of '52 or Big Smoke[1] was a severe air-pollution event that affected London during December 1952.
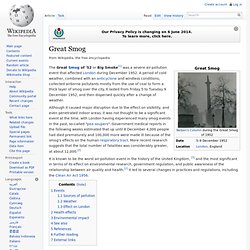
A period of cold weather, combined with an anticyclone and windless conditions, collected airborne pollutants mostly from the use of coal to form a thick layer of smog over the city. It lasted from Friday 5 to Tuesday 9 December 1952, and then dispersed quickly after a change of weather. Although it caused major disruption due to the effect on visibility, and even penetrated indoor areas, it was not thought to be a significant event at the time, with London having experienced many smog events in the past, so-called "pea soupers". Government medical reports in the following weeks estimated that up until 8 December 4,000 people had died prematurely and 100,000 more were made ill because of the smog's effects on the human respiratory tract.
More recent research suggests that the total number of fatalities was considerably greater, at about 12,000.[2] Motor vehicle emissions. Typical appearance of car exhaust on a correctly maintained and warmed car. 99.4% of pollutant gases are invisible[1] Motor vehicle emissions are composed of the by-products that comes out of the exhaust systems or other emissions such as gasoline evaporation.
These emissions contribute to air pollution and are a major ingredient in the creation of smog in some large cities.[2][3][4][5] A 2013 study by MIT indicates that 53,000 early deaths occur per year in the United States alone because of vehicle emissions.[6] According to another study from the same university, traffic fumes alone cause the death of 5000 people every year just in the United Kingdom.[7] Criteria air contaminants. Criteria air contaminants (CAC), or criteria pollutants, are a set of air pollutants that cause smog, acid rain, and other health hazards.
CACs are typically emitted from many sources in industry, mining, transportation, electricity generation and agriculture. Gas-s-s-s. Gas-s-s-s (also known as Gas!
Or It Became Necessary to Destroy the World in Order to Save It) is a 1971 motion picture produced and released by American International Pictures. It was producer Roger Corman's final film for AIP, after a long association. He was unhappy because AIP made several cuts to the film without his approval, including removing the final shot where God commented on the action - a shot which Corman regarded as one of the greatest he had made in his life.[1] The movie is a post-apocalyptic dark comedy, about survivors of an accidental military gas leak, of an experimental agent that kills everyone on Earth over the age of twenty-five. (A cartoon title sequence shows a John Wayne-esque Army General announcing — and denouncing — the "accident"; the story picks up after the victims have died.) Gas-s-s-s found a fresh airing on late night television in the 1980s, and was recently (2005) issued on DVD, as a double feature with Wild in the Streets, another AIP movie. Emission.
From Wikipedia, the free encyclopedia Emission may refer to:
Flue. A seven-flue chimney in a four storey Georgian house in London, showing alternative methods of sweeping Heat retention[edit] Flues are adjustable and are designed to release noxious gases to the atmosphere.
Sulfur dioxide. The specific person who discovered sulfur dioxide is not known, but it was first used by the Romans in winemaking, when they discovered that burning sulfur candles inside empty wine vessels keeps them fresh and free from vinegar smell.[2] Structure and bonding[edit] The sulfur - oxygen bond has a bond order of 1.5.
There is support for this simple approach that does not invoke d orbital participation.[3] In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence[edit] It is found on Earth and exists in very small concentrations and in the atmosphere at approximately 1 ppbv.[4][5] Production[edit] Sulfur dioxide is primarily produced for sulfuric acid manufacture (see contact process). Exhaust gas recirculation. History[edit] The first EGR systems were crude; some were as simple as an orifice jet between the exhaust and intake tracts which admitted exhaust to the intake tract whenever the engine was running.
Difficult starting, rough idling, and reduced performance and fuel economy resulted.[3] By 1973, an EGR valve controlled by manifold vacuum opened or closed to admit exhaust to the intake tract only under certain conditions. Control systems grew more sophisticated as automakers gained experience; Chrysler's "Coolant Controlled Exhaust Gas Recirculation" system of 1973 exemplified this evolution: a coolant temperature sensor blocked vacuum to the EGR valve until the engine reached normal operating temperature.[3] This prevented driveability problems due to unnecessary exhaust induction; NOx forms under elevated temperature conditions generally not present with a cold engine.
EGR in spark-ignited engines[edit] Reduced throttling losses. Reduced specific heat ratio.