

Synthemax plates. Lp_cellculture_selection_guide_3_02_lslp_cc_006.pdf (application/pdf Object) Corning[reg] Synthemax[trade] Surface: a tool for feeder-free, xeno-free culture of human embryonic stem cells : Article : Nature Methods. Corning® Cell Culture Surfaces: Synthemax Surface. The Corning Synthemax surface is designed to mimic a cells' natural environment with extra cellular matrix derived cell adhesion promoting peptides. This surface allows expansion and differentiation of embryonic stem cells for more than 20 serial passages in defined xeno-free medium with: Stable doubling time and cell viability Retention of phenotypic marker expression such as Oct4, TRA-1-60, SSEA4 and cell morphology (Figure 1) Retention of normal karyotype Retention of pluripotency as determined by embryoid body formation and teratomas Directed differentiation into specific tissue types such as cardiomyocytes (Figure 2) Synthetic, xeno-free substrate Gamma sterilized (SAL 10-6) Quality tested for lot-to-lot consistency and traceability Stored at room temperature for 2 years Ready to use Available in multiple formats: 6 well plates and T-75 flasks.
STO feeder cells. Mitomycin C. Frequently Asked Questions are available for this Product.
Application Mitomycin C is used to generate mitotically inactive feeder cells used in cell culture systems, such as mitotically inactive fibroblast used in embryonic stem cell (ESC) systems. Biochem/physiol Actions Inhibitor of DNA synthesis, nuclear division, and cancer cells. General description Mitomycin C is an anti-neoplastic antibiotic, DNA inter-strand, cross-linking, alkylating agent that targets guanine nucleoside in the sequence 5′CpG-3′.
Other Notes Vial contains 2 mg mitomycin C and 48 mg NaCl. Protocols & Applications Antibiotic Selector for application, solubility, solution stability, working concentration, and mode of action information. Mitomycin. The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus or Streptomyces lavendulae.[1] One of these compounds, mitomycin C, finds use as a chemotherapeutic agent by virtue of its antitumour antibiotic activity.
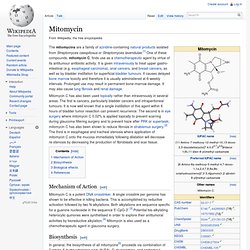
It is given intravenously to treat upper gastro-intestinal (e.g. esophageal carcinoma), anal cancers, and breast cancers, as well as by bladder instillation for superficial bladder tumours. It causes delayed bone marrow toxicity and therefore it is usually administered at 6-weekly intervals. Prolonged use may result in permanent bone-marrow damage. It may also cause lung fibrosis and renal damage. Mitomycin C has also been used topically rather than intravenously in several areas. Mechanism of Action[edit] Mitomycin C is a potent DNA crosslinker. Biosynthesis[edit] Synthesis of the key intermediate, 3-amino-5-hydroxy-benzoic acid. Biological effects[edit] References[edit] Irr. STO cells. YS001 mouse stem cells. Catalog Search. References Doetschman TC, et al.
The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Williams RL, et al. NIH:Stem Cell Info. Appendix B: Mouse Embryonic Stem Cell Cultures. The techniques for culturing mouse embryonic stem (ES) cells from the inner cell mass of the preimplantation blastocyst were first reported 20 years ago [6, 11], and versions of these standard procedures are used today in laboratories throughout the world.
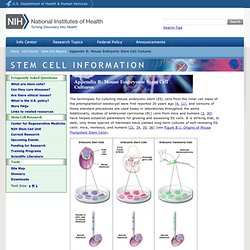
Additionally, studies of embryonal carcinoma (EC) cells from mice and humans [2, 30] have helped establish parameters for growing and assessing ES cells. It is striking that, to date, only three species of mammals have yielded long-term cultures of self-renewing ES cells: mice, monkeys, and humans [21, 34, 35, 36] (see Figure B.1.
Origins of Mouse Pluripotent Stem Cells). Figure B.1. Origins of Mouse Pluripotent Stem Cells. (© 2001 Terese Winslow) In mice, the efficiency of generating ES cells is influenced by the genetic strain of laboratory mice and individual factors that affect pregnant females. Another influence on the efficiency with which ES cells can be cultured from mouse blastocysts is the pregnancy status of the female. 2010 Articles.